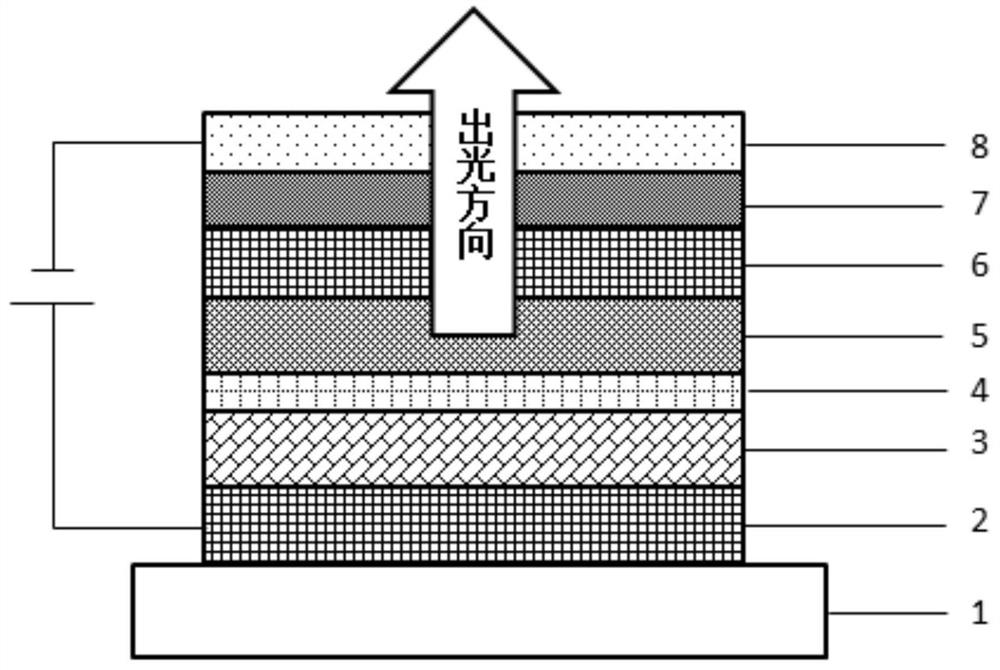
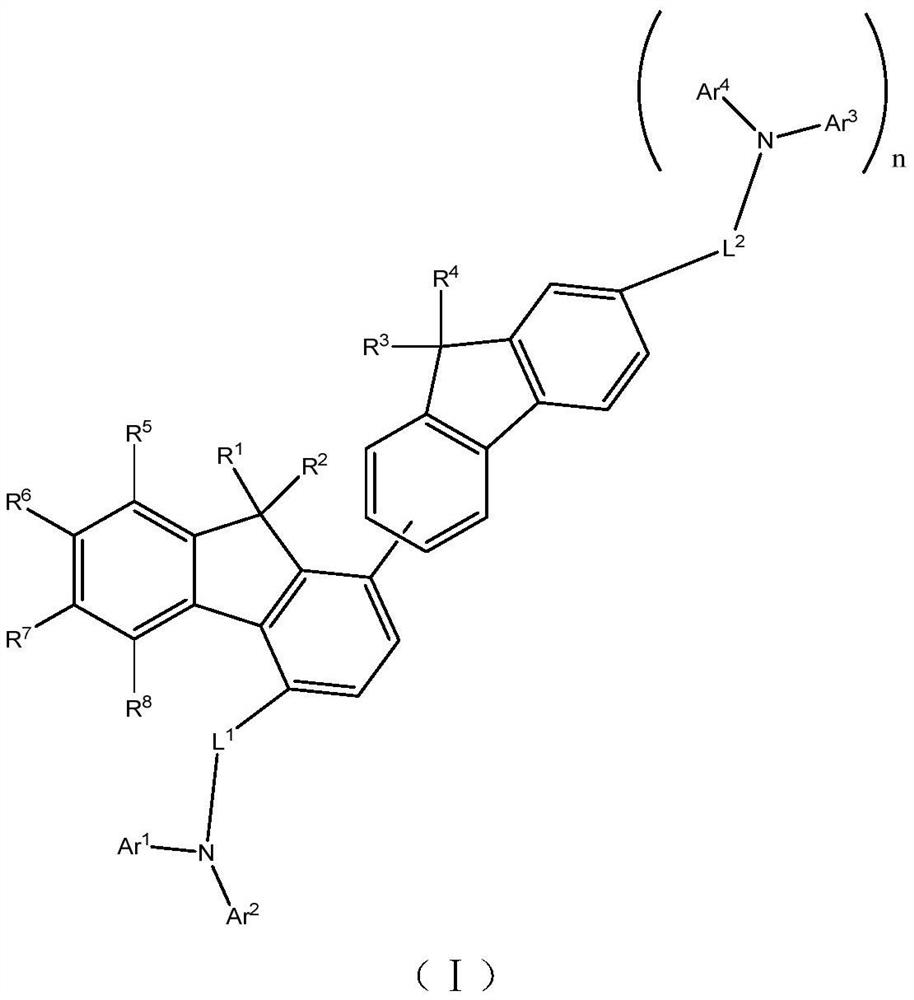
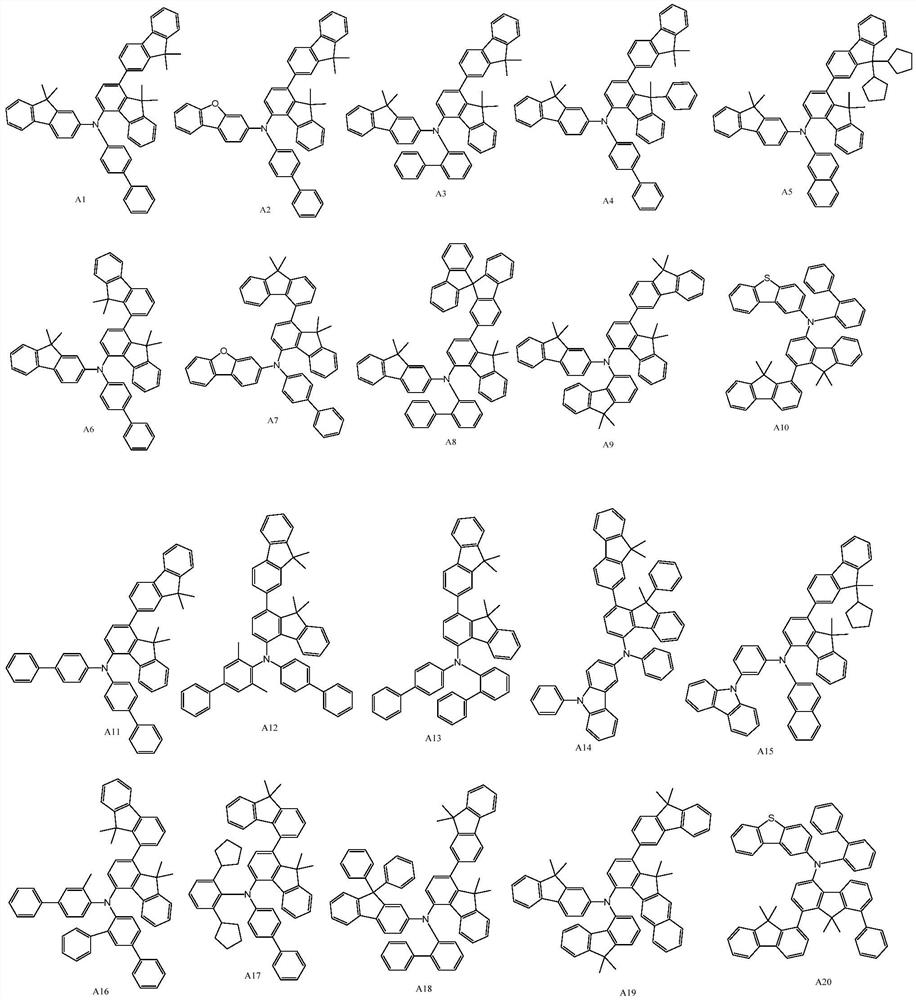
Compound, hole transport material and organic electroluminescent device
A compound and an independent technology, applied in the field of organic light-emitting display, can solve the problems that restrict the display function and development of OLED display devices, the efficiency and life cannot be balanced, and the hole migration rate is low, so as to achieve good thermodynamic stability and good luminous efficiency , The effect of simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0095] Synthesis Example 1: Synthesis of Compound A1
[0096]
[0097] Add 100mmol of 2-boronic acid methyl benzoate, 100mmol of 2-bromo-4-chlorophenol, 41.4g of potassium carbonate (300mmol), 800ml of tetrahydrofuran (THF) and 200ml of water in the reaction flask, and add 1mol% tetrahydrofuran (THF) (Triphenylphosphine) palladium (Pd(PPh 3 ) 4 ). React at 120°C for 12h. After the reaction was completed, the reaction was stopped, the reactant was cooled to room temperature, water was added, and the organic phase was concentrated to obtain a white solid, which was filtered and washed with water. The obtained solid was recrystallized and purified with toluene to obtain a white powder M1. Among them, Pd(PPh 3 ) 4 The added amount of 2-bromo-4-chlorophenol is 1mol%.
[0098] Add 100mmol of M1 and 200ml of THF into the reaction flask, add 220mmol of methylmagnesium bromide dropwise at 0°C, after the dropwise addition is completed, rise to room temperature and react for 12h...
Synthetic example 2
[0105] Synthesis Example 2: Synthesis of Compound A5
[0106]
[0107] Add 100mmol of 2-boronic acid methyl benzoate, 100mmol of 2-bromo-4-chlorophenol, 41.4g of potassium carbonate (300mmol), 800ml of THF and 200ml of water, and add 1mol% of Pd(PPh 3 ) 4 . React at 120°C for 12h. After the reaction was completed, the reaction was stopped, the reactant was cooled to room temperature, water was added, and the organic phase was concentrated to obtain a white solid, which was filtered and washed with water. The obtained solid was recrystallized and purified with toluene to obtain a white powder M1. Among them, Pd(PPh 3 ) 4 The added amount of 2-bromo-4-chlorophenol is 1mol%.
[0108] Add 100mmol of M1 and 200ml of THF into the reaction flask, add 220mmol of methylmagnesium bromide dropwise at 0°C, after the dropwise addition is completed, rise to room temperature and react for 12h. After the reaction is complete, water is added, the organic phase is separated and concent...
Synthetic example 3
[0115] Synthesis Example 3: Synthesis of Compound A7
[0116]
[0117] Add 100mmol of 2-boronic acid methyl benzoate, 100mmol of 2-bromo-4-chlorophenol, 41.4g of potassium carbonate (300mmol), 800ml of tetrahydrofuran (THF) and 200ml of water in the reaction flask, and add 1mol% tetrahydrofuran (THF) (Triphenylphosphine) palladium (Pd(PPh 3 ) 4 ). React at 120°C for 12h. After the reaction was completed, the reaction was stopped, the reactant was cooled to room temperature, water was added, and the organic phase was concentrated to obtain a white solid, which was filtered and washed with water. The obtained solid was recrystallized and purified with toluene to obtain a white powder M1. Among them, Pd(PPh 3 ) 4 The added amount of 2-bromo-4-chlorophenol is 1mol%.
[0118] Add 100mmol of M1 and 200ml of THF into the reaction flask, add 220mmol of methylmagnesium bromide dropwise at 0°C, after the dropwise addition is completed, rise to room temperature and react for 12h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com