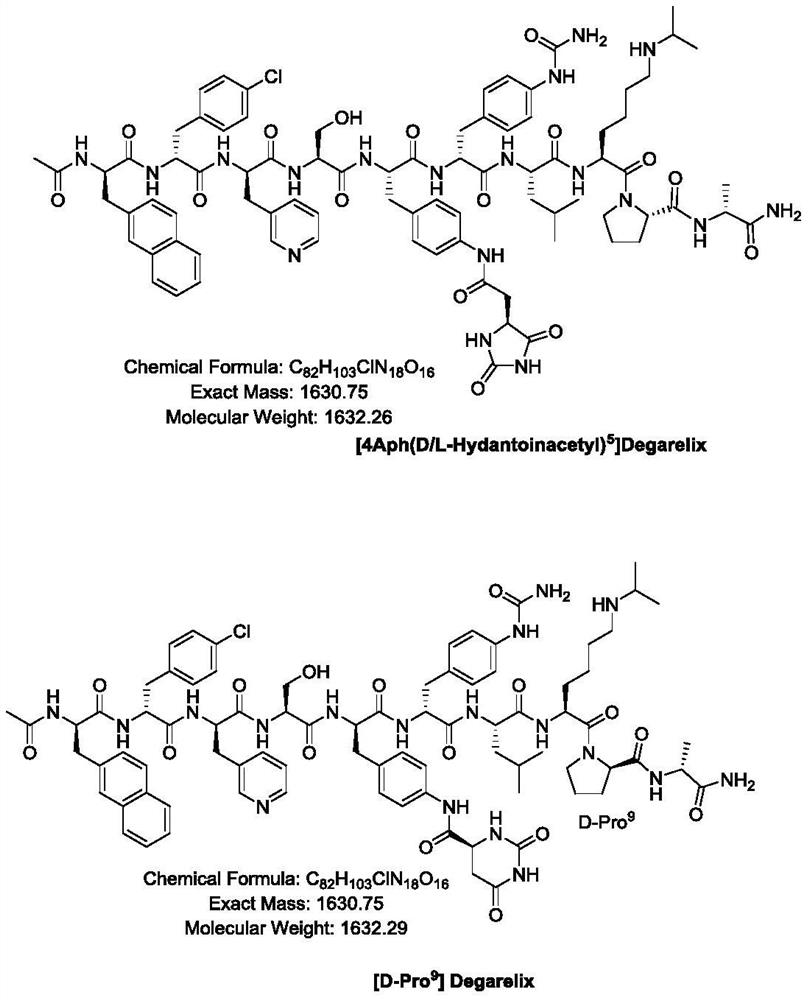
Preparation method of Degarelix
A degarelix and resin technology, which is applied in the field of degarelix preparation, can solve the problems that cannot be effectively avoided, the process is cumbersome, and the process is unstable, and achieves the effects of easy quality control, simple operation and little environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1Fmoc-Pro-D-Ala-Rink MBHA resin
[0038]Weigh Rink Amide MBHA resin (75g, 30mmol, degree of substitution: 0.40mmol / g) into a solid-phase reaction synthesis column. Add 500ml DMF to swell for 30min, and remove the DMF. The resin was washed with 3×400ml DMF, and the DMF was sucked off. Add 400 ml of DBLK solution (20% morpholine / DMF solution, v / v), deprotect twice, the first time is 5 min, the second time is 15 min. After deprotection, wash the resin with 400ml DMF each time for 6 times. After the 4th washing, pick a little resin with a glass rod, and the ninhydrin test is positive, indicating that Fmoc has been removed.
[0039] Weigh 40.24g Fmoc-Pro-D-Ala-OH and 9.73g HOBt, add 120ml DMF to dissolve, after complete dissolution, cool the solution below 5°C, then add 11.36g DIC (pre-cooled to <0°C), Activate in the solution for about 4 minutes, control the activated solution into the reaction column, react at 20-30°C for 2 hours, the ninhydr...
Embodiment 2
[0040] Synthesis of embodiment 2 degarelix resin
[0041] Weigh the Fmoc-Pro-D-Ala-Rink MBHA resin (30 mmol) obtained in Example 1 into a solid-phase reaction synthesis column. The resin was washed with 3×400ml DMF, and the DMF was sucked off. Add 400 ml of DBLK solution (20% morpholine / DMF solution, v / v), deprotect twice, the first time is 5 min, the second time is 15 min. After deprotection, wash the resin with 400ml DMF each time for 6 times. After the 4th washing, pick a little resin with a glass rod, and the Chloranil test is positive, indicating that Fmoc has been removed.
[0042] Weigh 30.64g Fmoc-ILys(Boc)-OH and 9.73g HOBt, add 120ml DMF to dissolve, after complete dissolution, cool the solution below 5°C, then add 11.36g DIC (pre-cooled to <0°C), Activate in the solution for about 4 minutes, add the activated solution to the reaction column, react at 20-30°C for 2 hours, Chloranil test is negative, remove the reaction solution, add 400ml of DMF to wash the resin, ...
Embodiment 3
[0048] Example 3 Cleavage of Degarelix Resin
[0049] Take 1100ml of frozen lysate (not higher than 10°C), the ratio is TFA:TIS::H 2 O=92.5:2.5:2.5 (v / v / v), add 110g (28mmol) of the degarelix peptide resin obtained in Example 2, react at 20-30°C for 2.0h, filter with suction, wash the resin with TFA, and concentrate the filtrate to 1 / 2-2 / 3 volume, then add 10 times the volume of isopropyl ether to settle, centrifuge to collect the precipitate, wash with isopropyl ether 6 times, and dry in vacuum to obtain 41.38 g of crude peptide, with a yield of 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com