Quercetin-low molecular heparin-paclitaxel conjugate as well as preparation method and application thereof
A low-molecular-weight heparin and quercetin technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc., can solve the problems of instability, limitation, and low water solubility of quercetin. , to achieve the effect of mild conditions and good versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The second aspect of the present invention provides the preparation method of the paclitaxel-low molecular weight heparin-quercetin conjugate described in the first aspect, the preparation method comprising the following steps: respectively synthesizing adipate dihydrazide modified low molecular weight heparin (LWMH- ADH), quercetin-NHS ester (QT-NHS) and carboxylated paclitaxel (PTX-COOH), using adipate dihydrazide modified low molecular weight heparin and quercetin-NHS ester as raw materials to synthesize low molecular weight heparin-quercetin Cortex (LWMH-QT); the paclitaxel-low molecular weight heparin-quercetin conjugate (PTX-LWMH-QT) is obtained by mixing and reacting low molecular weight heparin-quercetin and carboxylated paclitaxel.
[0032] Preferably, the synthesis method of adipate dihydrazide modified low-molecular-weight heparin is as follows: add adipamide (ADH), EDCI, HOBt to the aqueous solution of low-molecular-weight heparin, add lye to adjust the pH va...
Embodiment 1
[0098] The preparation of embodiment 1 PTX-LWMH-QT
[0099]1. Synthesis of LWMH-QT
[0100] (1) Weigh 0.25g of LWMH and dissolve it in 50mL of distilled water, stir to dissolve it fully, then add 4g of ADH, 0.48g of EDCI, and 0.48g of HOBt. Adjust the pH of the system to 6.8 with 0.1M NaOH solution, stir and react at room temperature for 24 hours to obtain a crude product, dialyze against water for 3 days, change the water every 6 hours, filter the dialysate and freeze-dry to obtain LWMH-ADH.
[0101] (2) Weigh 302 mg of QT and 200 mg of succinic anhydride, dissolve them together in 10 mL of tetrahydrofuran, add 805 μL of pyridine, and react under nitrogen protection for 3 days. The solvent in the reaction solution was removed by rotary evaporation, washed with alkali and saturated brine, dried, and passed through a silica gel column to obtain the intermediate quercetin hemisuccinic acid.
[0102] (3) Dissolve 1.15g of N-hydroxysuccinimide in 6mL of dichloromethane, add 1.4m...
Embodiment 2
[0108] Example 2 Preparation of PTX-LWMH-QT self-assembled nanoparticles
[0109] Weigh 5 mg of PTX-LWMH-QT, disperse it in 3 mL of PBS (pH=7.4, 10 mM), stir well and then ultrasonicate the sample (40 W, 2 s on / 4 s off, 4 min in total). After the ultrasonic sample is filtered through a 0.8 μm microporous membrane, the PTX-LWMH-QT self-assembled nanoparticle solution can be obtained.
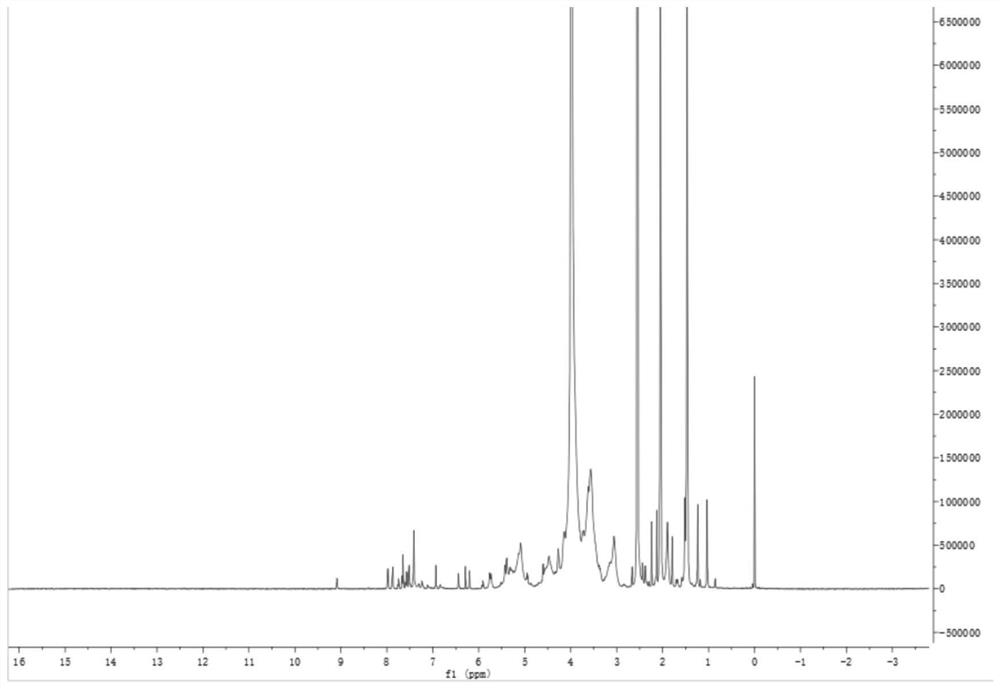
[0110] Weigh 10mg PTX-LWMH-QT, use d 6 -DMSO / D 2 O mixed solvent to dissolve it, carry out 1 H-NMR analysis, get figure 2 As shown in the spectrum, the characteristic absorption peaks of PTX and QT appeared, proving that the polymer was successfully synthesized.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com