Method for gas-phase epoxidation reaction of olefin and hydroperoxide
A technology of epoxidation reaction and hydroperoxide, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problem that hydroperoxide cannot be completely reacted, the heat of epoxidation reaction cannot be used, and the production cost of epoxide can be reduced etc. to achieve the effects of reducing the amount of decomposition, increasing the utilization rate of energy, increasing the reaction rate and conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0049] Implementation 2: 0.5MPa, with nitrogen as the carrier gas, 30% (wt) hydrogen peroxide is atomized and gasified at 140°C, the residence time in the gas phase is 1 second, and then quenched to room temperature, and the decomposition rate of hydrogen peroxide is tested, as shown in Table 2:
[0050] serial number h 2 o 2 Concentration before gasification (%)
h 2 o 2 Concentration after gasification (%)
concentration change Decomposition rate (%) 1 10.448 10.082 0.335 3.508 2 8.432 8.291 0.198 1.667 3 6.509 6.468 0.096 0.624 4 5.043 5.030 0.049 0.247 5 4.096 4.091 0.029 0.119 6 3.114 3.112 0.013 0.041 7 2.303 2.302 0.007 0.016 8 1.112 1.110 0.002 0.002
[0051] Table 2 is the hydrogen peroxide vapor phase decomposition rate table.
[0052] It can be seen that, in the case of controlling the residence time, the decomposition rate of hydrogen peroxide produced by high temperature...
Embodiment 1
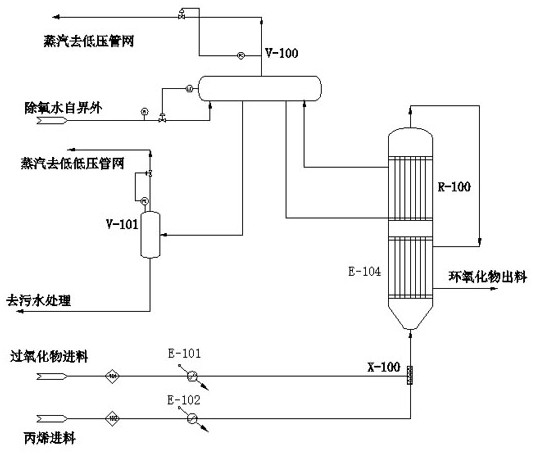
[0054] Example 1, such as figure 1 As shown, the equipment involved in this system is mainly an integrated reactor. In this embodiment, the integrated reactor is divided into two sections, the lower section is a shell-and-tube heat exchange section, and the upper section is a tube-and-tube reaction section.
[0055] Such as figure 1 As shown, this embodiment relates to a method for gas-phase epoxidation of propylene and hydrogen peroxide, and the specific process is as follows: proportioning raw materials according to the molar ratio of propylene and hydrogen peroxide in a ratio of 1:6, and the concentration of hydrogen peroxide is 30% ( wt); at 1.0MPa, gasify propylene and preheat to 120°C, add hydrogen peroxide into the gaseous propylene stream, and preheat the hydrogen peroxide to 10°C below the self-accelerating decomposition temperature SADT to reduce the amount of thermal decomposition of hydrogen peroxide. The temperature is slightly different for different peroxides; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com