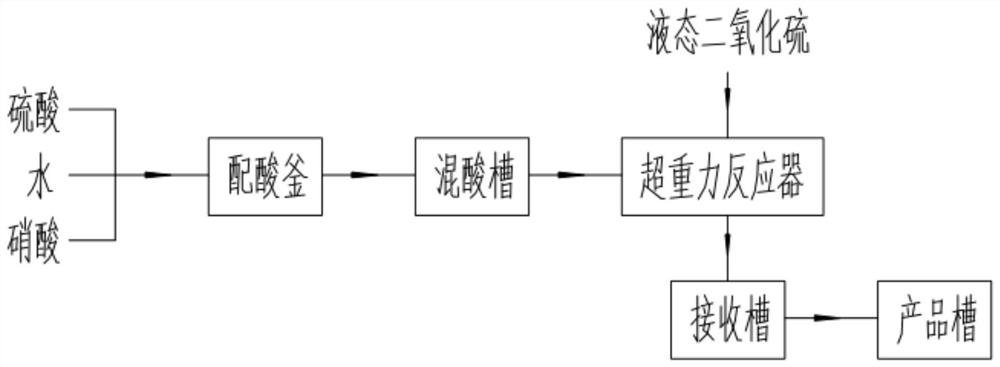
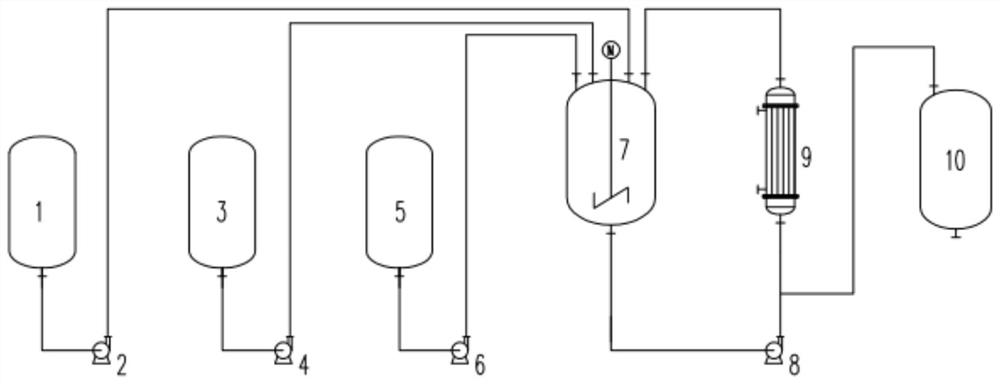
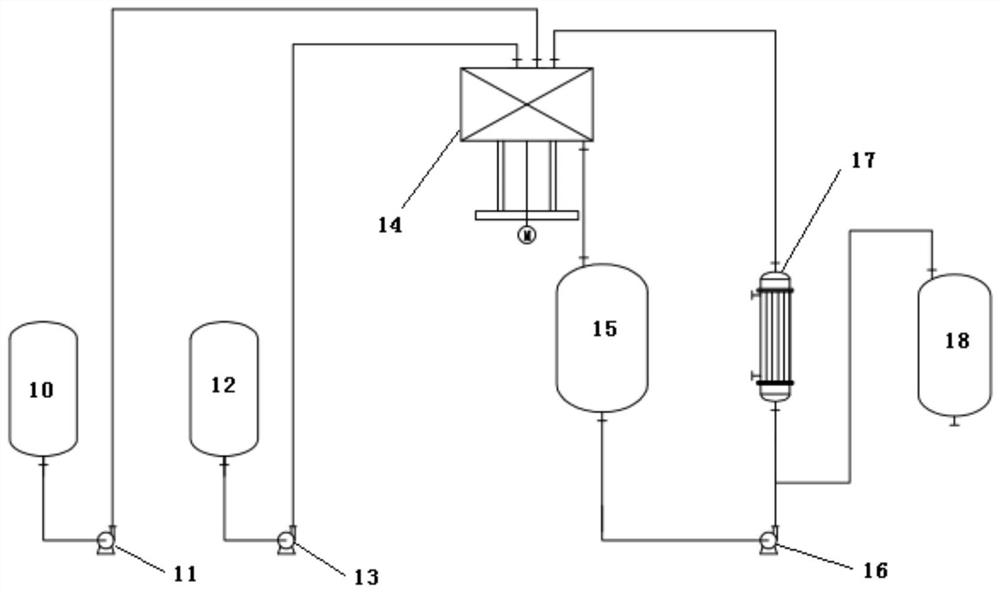
A method and system for continuous production of nitrosyl sulfuric acid
A technology of nitrosylsulfuric acid and sulfuric acid, applied in chemical instruments and methods, inorganic chemistry, nitrogen compounds, etc., can solve the problems of inaccurate sulfur dioxide gas feed, easy decomposition of nitrosylsulfuric acid, high reaction temperature, etc., to achieve sulfur dioxide Less loss, less decomposition, and higher reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] In this embodiment, sulfuric acid (98% by mass), nitric acid (98% by mass), liquid sulfur dioxide and water are used as raw materials.
[0059] (1) The feed flow rate of sulfuric acid is 723.9kg / h, the feed flow rate of water is 110.8kg / h, and the feed flow rate of nitric acid is 165.3kg / h, which are continuously added to the acid mixing tank, and the temperature of the acid mixing tank is controlled at 30°C. The mass fraction of nitric acid in the mixed acid is 16.20%.
[0060] (2) The feed flow rate of liquid sulfur dioxide is 164.57kg / h, the feed flow rate of mixed acid is 1000kg / h, continuously enters the supergravity reactor, the pressure of the supergravity reaction system is controlled at 0.5MPa, and the output of the supergravity reactor flows into the receiving tank , the temperature of the entire reaction cycle system is controlled at 50° C., and the average residence time of the reaction materials in the reaction cycle system is 20 min. After continuous reac...
Embodiment 2
[0062] In this embodiment, nitration waste sulfuric acid (mass content 87%), nitric acid (mass content 98%), and liquid sulfur dioxide are used as raw materials.
[0063] (1) The feed flow rate of sulfuric acid is 1194kg / h, and the feed flow rate of nitric acid is 306kg / h, which is continuously added to the mixed acid tank, and the temperature of the acid mix tank is controlled at 30°C, and the mass fraction of nitric acid in the mixed acid obtained by configuring is 19.99%.
[0064] (2) The feed flow rate of liquid sulfur dioxide is 304.64kg / h, the feed flow rate of mixed acid is 1500kg / h, continuously enters the supergravity reactor, the pressure of the supergravity reaction system is controlled at 0.5MPa, and the output of the supergravity reactor flows into the receiving tank , the temperature of the entire reaction cycle system is controlled at 50° C., and the average residence time of the reaction materials in the reaction cycle system is 20 min. After continuous reactio...
Embodiment 3
[0066] In this embodiment, sulfuric acid (98% by mass), nitric acid (98% by mass), liquid sulfur dioxide and water are used as raw materials.
[0067] (1) The feed flow rate of sulfuric acid is 1377kg / h, the feed flow rate of water is 223kg / h, and the feed flow rate of nitric acid is 500kg / h, which are continuously added to the mixed acid tank, and the temperature of the acid mix tank is controlled at 30°C. The mass fraction of nitric acid is 24.5%.
[0068] (2) The feed flow rate of liquid sulfur dioxide is 497.78kg / h, the feed flow rate of mixed acid is 2000kg / h, continuously enters the supergravity reactor, the pressure of the supergravity reaction system is controlled at 0.5MPa, and the discharge of the supergravity reactor flows into the receiving tank. The temperature of the entire reaction cycle system is controlled at 50° C., and the average residence time of the reaction materials in the reaction cycle system is 20 minutes. After continuous reaction for 10 hours, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com