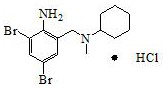
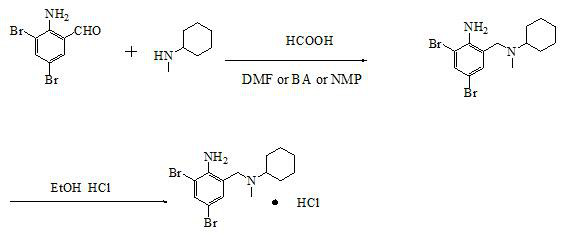
Synthetic method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and a synthesis method, which is applied in the field of chemical synthesis, can solve the problems of strong corrosiveness of workshop equipment, difficult to handle effectively, and is not suitable for large-scale production, and achieves the effects of cost reduction, convenient operation and little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 27.9g 2-amino-3,5-dibromobenzaldehyde, 12.5gN-methylcyclohexylamine, 0.84g% 5 palladium carbon and 55g butyl acetate in a 500ml reaction flask and heat up to 100°C, then add dropwise Water formic acid 9.2g, after 1.5h dropwise addition, keep warm at 100-110°C and react for 5h, cool down to 30-35°C, press filter, concentrate the filtrate under reduced pressure until no solvent is evaporated, cool down to 25-30°C, add 25g30 % hydrogen chloride ethanol solution, temperature control 30-35 ℃, stirring reaction 2h-3h, suction filtration, filter cake with 5 times the weight of methanol acetone mixed solution (W methanol: W acetone = 5:1) recrystallization, to obtain the product 37.6kg, yield 91.3%, HPLC purity 99.1%.
Embodiment 2
[0036] Add 27.9g 2-amino-3,5-dibromobenzaldehyde, 12.5gN-methylcyclohexylamine, 0.84g%5 palladium carbon and 55g N,N-dimethylformamide in the 500ml reaction flask and heat up to 100 ℃, add 9.2 g of anhydrous formic acid dropwise, after 1.5 hours of dropping, keep warm at 100-110 ℃ for 5 hours, cool down to 30-35 ℃, press filter, concentrate the filtrate under reduced pressure until no solvent is evaporated, and cool down to 25 -30°C, add 25g of 30% hydrogen chloride ethanol solution, control the temperature at 30-35°C and stir for 2h-3h, filter with suction, and use 5 times the weight of methanol-acetone mixed solution for the filter cake (W methanol:W acetone=5:1) After recrystallization, 37.8kg of the product was obtained, the yield was 91.6%, and the HPLC purity was 99.4%.
Embodiment 3
[0038] Add 27.9kg of 2-amino-3,5-dibromobenzaldehyde, 12.5kg of N-methylcyclohexylamine, 840g of 5% palladium carbon and 55kg of N,N-dimethylformamide into a 100L reaction flask and heat up to 100°C , add 9.2kg of anhydrous formic acid dropwise, after 1.5h of dropwise addition, keep warm at 100-110°C for 5h, cool down to 30-35°C, press filter, concentrate the filtrate under reduced pressure until no solvent is evaporated, cool down to 25-30°C ℃, after adding 25kg of 30% hydrogen chloride ethanol solution, control the temperature at 30-35℃ and stir the reaction for 2h-3h, filter with suction, and recrystallize the filter cake with 5 times the weight of methanol acetone mixed solution (W methanol: W acetone = 5:1) , to obtain product 38.1kg, yield 92.3%, HPLC purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com