Novel method for synthesizing vitamin K2
A synthetic method and vitamin technology, applied in the field of medicine and fine chemical industry, can solve problems such as difficult operation, dangerous reaction reagents, low synthesis yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
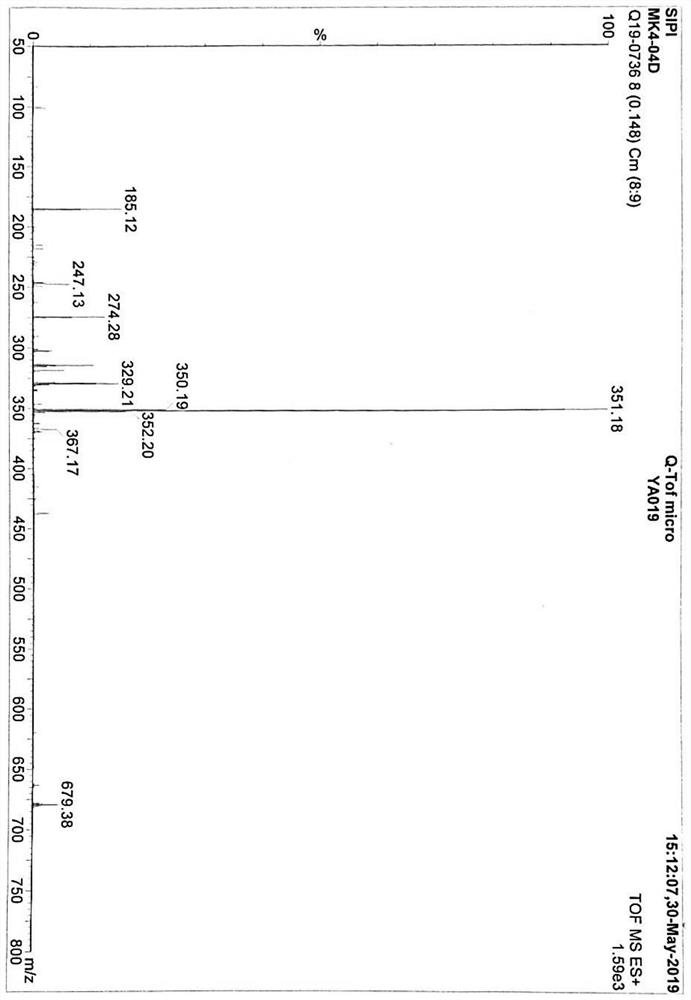
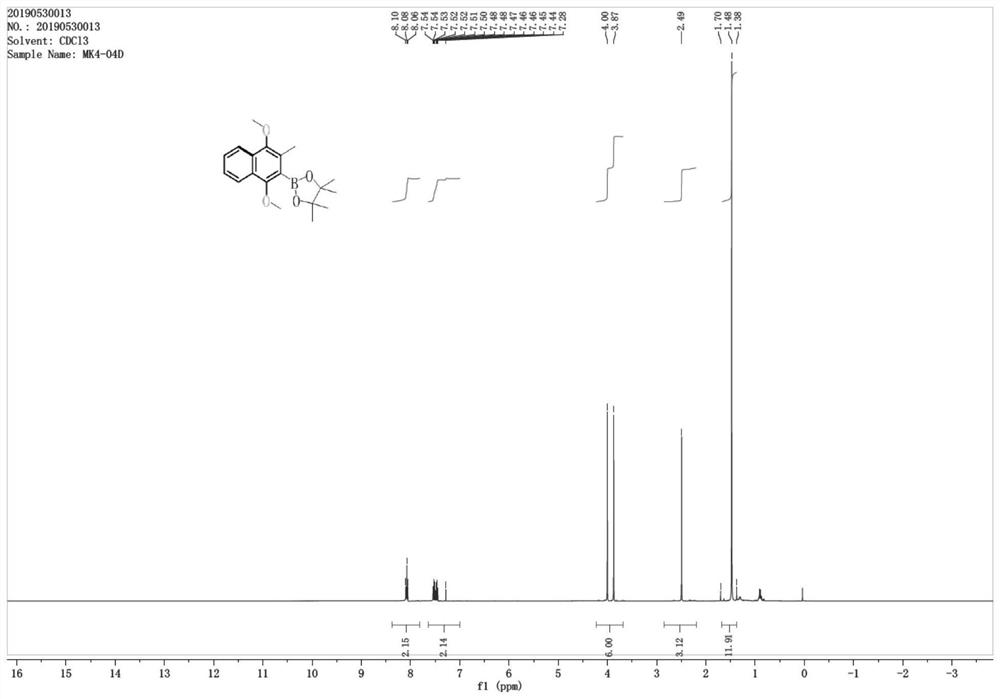
[0064] In the preparation method of vitamin K2 of the present invention, a structurally new intermediate is provided, namely the compound shown in formula 4,
[0065]
[0066] Its chemical name is 2-(1,4-dimethoxy-3-methyl-2-naphthalene)-4,4,5,5-tetramethyl-1,3,2-dioxaborane. Utilizing the intermediate, the yield of the final product vitamin K2 is significantly improved, and the whole synthesis process is simple and safe in operation.
[0067] Advantages of the present invention:
[0068] 1. The present invention provides a brand-new synthetic method for vitamin K2;
[0069] 2. The synthetic method of the present invention is simple to operate and easy to industrialized production;
[0070] 3. the yield of the vitamin K2 of synthetic method of the present invention is high;
[0071] 4. the reaction conditions of synthetic method of the present invention are gentle;
[0072] 5. The synthesis method of the present invention is friendly to the environment and operators.
Embodiment 1
[0075] Embodiment 1: Synthesis of the compound of formula 8.
[0076]
[0077] Referring to the method disclosed in WO2011 / 117324, the present inventors synthesized the compound of formula 8. The specific steps are as follows:
[0078] Add 28.2 g of 2-bromo-1,4-dimethoxy-3-menaphthoquinone (Formula 3) and 200 ml of tetrahydrofuran into the reaction flask, cool down to -78°C under the protection of nitrogen, and dropwise add 100 ml of 1M n-butyl Lithium-based n-hexane solution, stirred for 60 minutes after dropping, added 18.8 g of triisopropyl borate dropwise under temperature control at -78°C, reacted for 30 minutes after dropping, warmed up to room temperature, quenched with 1N hydrochloric acid solution, extracted with isopropyl ether, and the organic layer was The neutral was washed with saturated brine, the organic layer was dried over sodium sulfate and filtered, and the filtrate was evaporated to remove the solvent to obtain a residue of 14.6 g. The residue was pur...
Embodiment 2
[0079] Embodiment 2: synthetic formula 7 compounds
[0080]
[0081] Referring to the method disclosed in WO2011 / 117324, the present inventors synthesized the compound of formula 7 from the compound of formula 8. The specific steps are as follows:
[0082] In 6 reaction flasks, add formula 8 compound 12.3g, DMF 150 milliliters, bromogeranyl linalool (formula 6) 17.7g, potassium phosphate 15.9g, then add palladium chloride 0.18g in No. 1 reaction flask, Add 0.18 g of nickel chloride to No. 2 reaction flask, add 0.5 g of tetrakistriphenylphosphine nickel to No. 3 reaction flask, add 0.5 g of tetrakistriphenylphosphine palladium to No. 4 reaction flask, and add dppfNiCl to No. 5 reaction flask 2 0.35g. Add dppfPdCl to No. 6 reaction bottle 2 0.35g. After the addition, the six reaction flasks were heated to an internal temperature of 70°C under the parallel heating module and reacted for 15 hours. After the reaction was completed, the temperature was lowered to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com