Preparation method of silver/silver chloride composite nanocube
A technology of nano-cubes and silver chloride, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., can solve problems such as complex preparation processes, achieve low reaction temperature, eliminate the use of organic solvents, and save costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Drug dosage:
[0024] Sodium lignosulfonate solution: 0mL
[0025] Sodium chloride solution: 10mL
[0026] Silver nitrate solution: 20mL
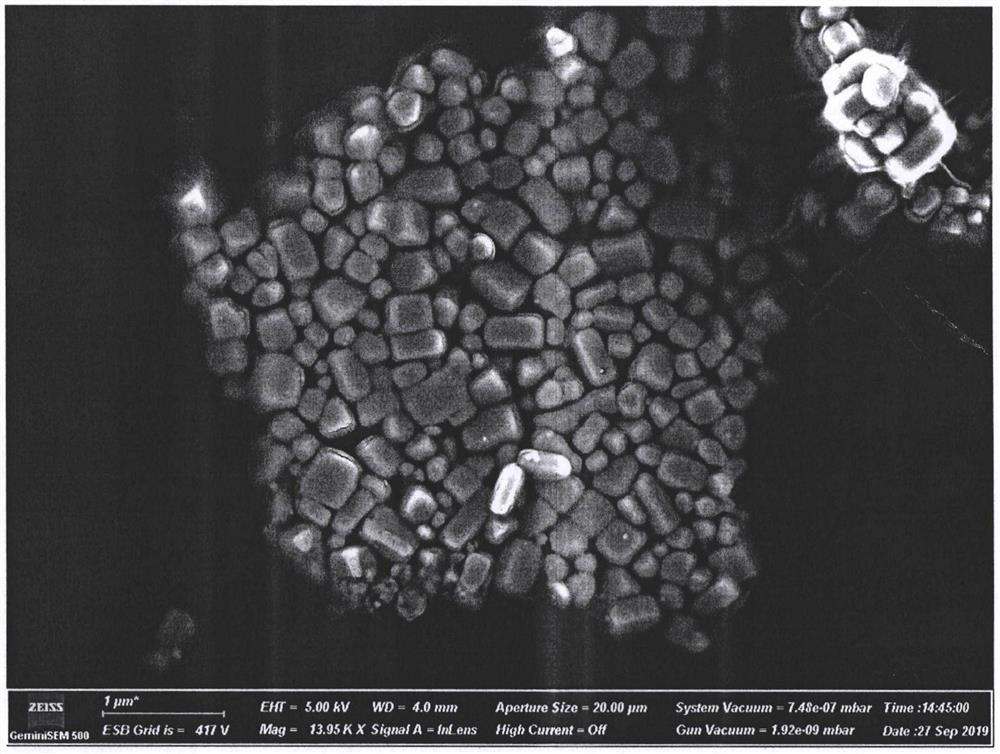
[0027] Measure 10mL of NaCl solution in a 50mL beaker and heat to 60□ under magnetic stirring. Then, 20 mL of silver nitrate solution was quickly poured into the beaker, and the reaction was stirred for 1 h. The Ag / AgCl colloidal solution that obtains uses deionized water to wash, and centrifuges 20min under 10000rpm, removes supernatant liquid, obtains silver / silver chloride composite nano cube particle after freeze-drying and carries out scanning electron microscope test (SEM) (test result See attached figure 1 )
Embodiment 2
[0029] Drug dosage:
[0030] Sodium lignosulfonate solution: 25mL
[0031] Sodium chloride solution: 10mL
[0032] Silver nitrate solution: 20mL
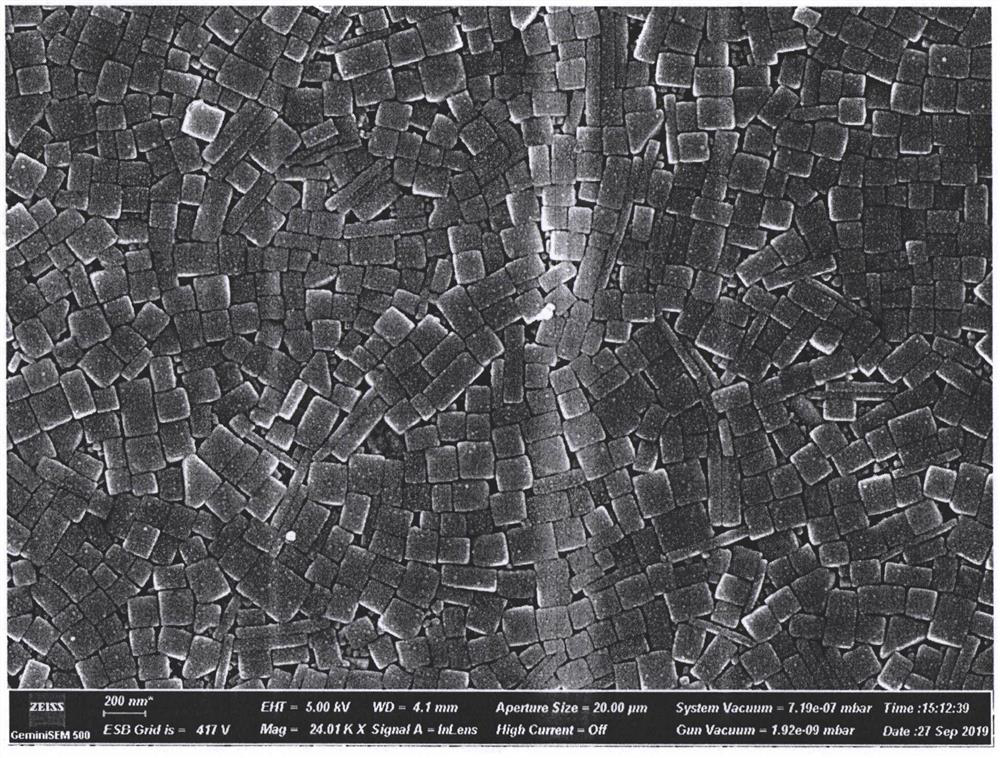
[0033] Measure 25mL of sodium lignosulfonate solution and 10mL of sodium chloride solution in a beaker, mix evenly under magnetic stirring, and heat to 60□; then measure 20mL of silver nitrate solution and quickly inject into the mixture, and continue to stir and react at 60□ 1h, forming a silver / silver chloride nanocomposite cube colloid solution; washing the prepared colloid solution with deionized water, and centrifuging at 10000rpm for 20min, removing the upper liquid, and obtaining silver / silver chloride composite nanocubes after freeze-drying Particles were subjected to scanning electron microscopy (SEM) (see the attached instructions for test results) figure 2 ).
Embodiment 3
[0035] Drug dosage:
[0036] Sodium lignosulfonate solution: 50mL
[0037] Sodium chloride solution: 10mL
[0038] Silver nitrate solution: 20mL
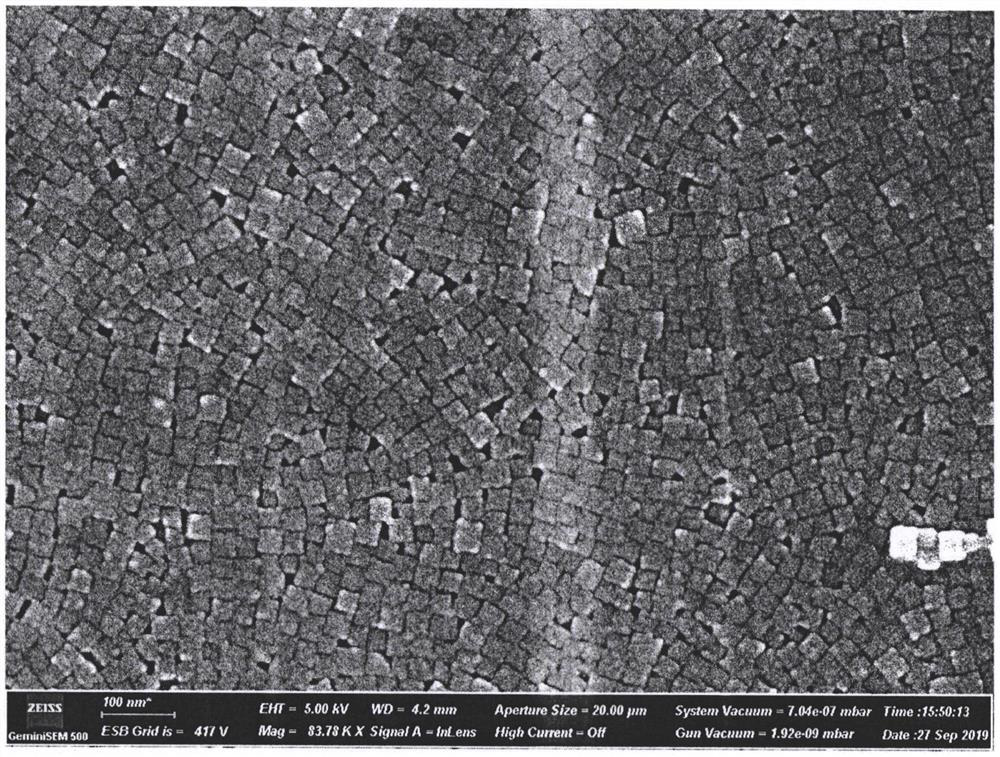
[0039] Measure 50mL of sodium lignosulfonate solution and 10mL of sodium chloride solution in a beaker under magnetic stirring, mix evenly, and heat to 60°C; then measure 20mL of silver nitrate solution and quickly inject into the mixture, and continue stirring reaction at 60°C 1h, forming a silver / silver chloride nanocomposite cube colloid solution; washing the prepared colloid solution with deionized water, and centrifuging at 10000rpm for 20min, removing the upper liquid, and obtaining silver / silver chloride composite nanocubes after freeze-drying Particles were subjected to scanning electron microscopy (SEM) (see the attached instructions for test results) image 3 ).
[0040] The test results show that when no sodium lignosulfonate is added, although Ag / AgCl cubes can be obtained, the size is very large and non-uniform. Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com