Preparation method of enalapril maleate
A technology of enalapril maleate and maleic acid, applied in the field of preparation of enalapril maleate, can solve problems such as unfavorable industrialized production, increased production cost, long reaction time, etc., and achieves the advantages of avoiding toxic reagents. The effect of using, small amount of three wastes, and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
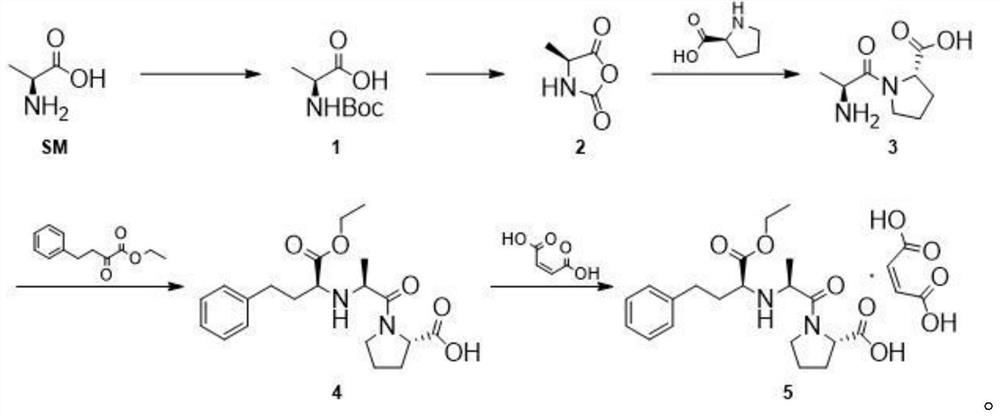
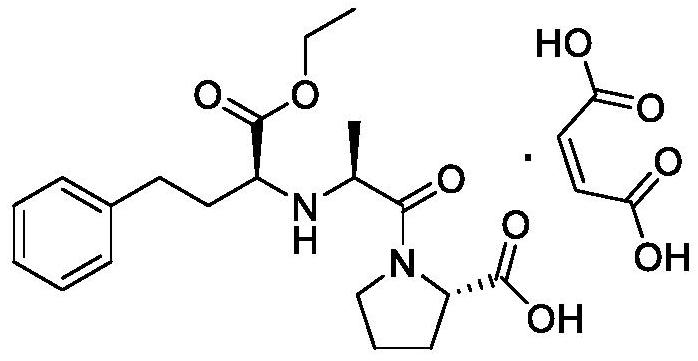
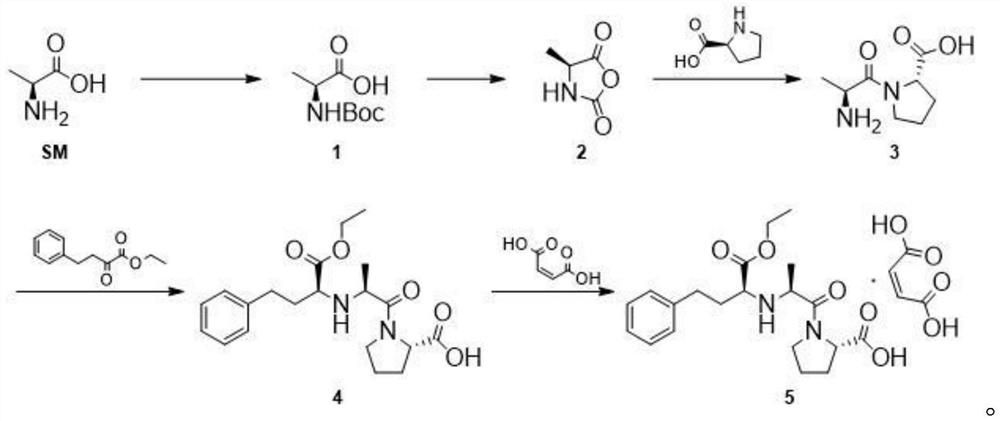
[0034] (1) Weigh 50g of L-alanine and 34g of sodium hydroxide into the reaction flask, add them into 300ml of purified water, start stirring, cool down to 0°C, and weigh 160g (Boc) 2 O, be dissolved in the THF of 300ml, control reaction temperature 0 ℃, (Boc) 2 The THF solution of O was added dropwise to the reaction system, and then the temperature was raised to 20° C. for 16 h. After the reaction, extract twice with petroleum ether 2*250ml, discard the organic phase, adjust the pH of the aqueous phase to about 2 with 10% hydrochloric acid, then extract four times with ethyl acetate 4*300ml, combine the organic phases, dry Dry over sodium sulfate, filter and concentrate to obtain intermediate 1.
[0035] (2) Measure 2L of dichloromethane, add it to a 5L three-necked flask, add 102g of intermediate 1 into the reaction system, protect it with nitrogen, cool down to -5°C, add 90g of phosphorus trichloride dropwise, and continue the reaction for 2 hours after the addition is com...
Embodiment 2
[0041] (1) Weigh 50g of L-alanine and 80g of potassium carbonate into the reaction flask, add them to 300ml of purified water, start stirring, cool down to 5°C, and weigh 180g (Boc) 2 O, be dissolved in the THF of 300ml, control reaction temperature 5 ℃, (Boc) 2 The THF solution of O was added dropwise into the reaction system, and then the temperature was raised to 30° C. for 24 h. After the reaction, extract twice with petroleum ether 2*250ml, discard the organic phase, adjust the pH of the aqueous phase to about 2 with 10% hydrochloric acid, then extract four times with ethyl acetate 4*300ml, combine the organic phases, dry Dry over sodium sulfate, filter and concentrate to obtain intermediate 1.
[0042] (2) Measure 2L of dichloromethane and add it to a 5L three-necked flask, add 102g of intermediate 1 into the reaction system, protect it under nitrogen, cool down to 5°C, add 90g of thionyl chloride dropwise, and continue the reaction for 2h after the addition. After the...
Embodiment 3
[0048] (1) Weigh 50g of L-alanine and 80g of potassium carbonate into the reaction flask, add them into 300ml of purified water, start stirring, cool down to 3°C, and weigh 180g (Boc) 2 O, be dissolved in the THF of 300ml, control reaction temperature 3 ℃, (Boc) 2 The THF solution of O was added dropwise into the reaction system, and then the temperature was raised to 25° C. for 24 h. After the reaction, extract twice with petroleum ether 2*250ml, discard the organic phase, adjust the pH of the aqueous phase to about 2 with 10% hydrochloric acid, then extract four times with ethyl acetate 4*300ml, combine the organic phases, dry Dry over sodium sulfate, filter and concentrate to obtain intermediate 1.
[0049] (2) Measure 2L of dichloromethane and add it to a 5L three-necked flask, add 102g of intermediate 1 into the reaction system, protect it under nitrogen, cool down to 5°C, add 100g of phosphorus oxychloride dropwise, and continue the reaction for 2 hours after the additi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com