Mesoporous sponge spicule as well as preparation method and application of mesoporous sponge spicule
A technology of sponge and bone spicules, applied in pharmaceutical formulations, medical preparations with non-active ingredients, inorganic non-active ingredients, etc., can solve the problems of insufficient convenience, reduced SHS penetration effect, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
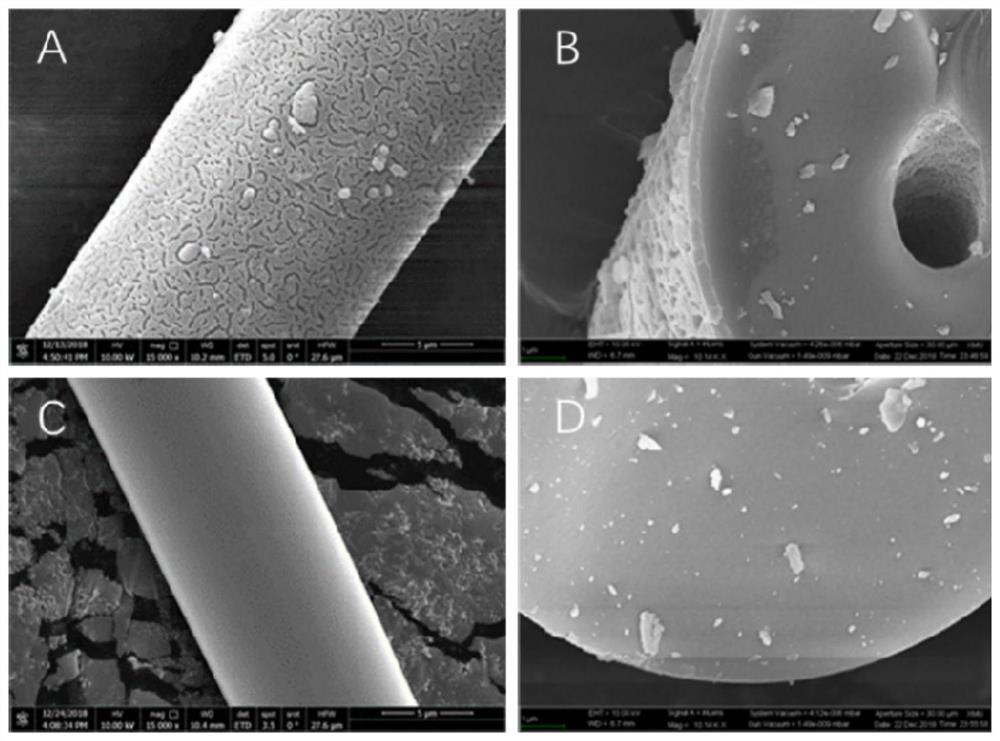
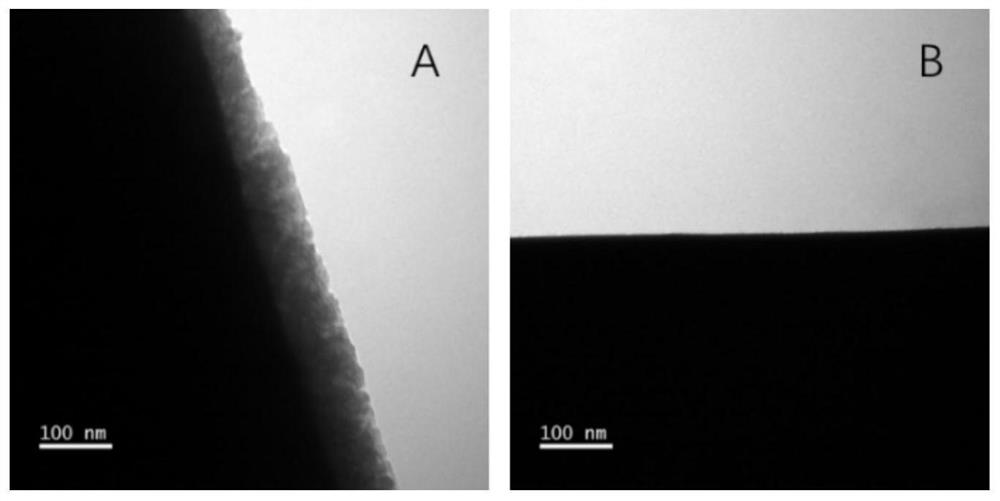
[0042] Mesoporous silica modified on the surface of bee sponge spicules
[0043]Bee sponge spicules: For the preparation method, see CN201610267764.6 "Preparation method of high-purity sponge spicules"
[0044] The following method can be used to prepare the surface-modified mesoporous silica of bee sponge spicules:
[0045] ①100mg of bee sponge spicules, add 35ml of deionized water, 80mg of cetyltrimethylammonium bromide (CTAB) or cetyltrimethylammonium chloride (CTAC), concentrated ammonia water (also hydrogen Sodium oxide or hydrochloric acid) (3-10ul, 20-50wt%), ethanol (10-100ml), stirred for 30min to form a uniform solution;
[0046] ② Add 30-100ul methyl orthosilicate (also can be ethyl orthosilicate or propyl orthosilicate) dropwise, then react statically at 30-100°C for 20-100 hours, wash with water to remove by-products, 100-120 Aging overnight at ℃;
[0047] ③ Remove the surfactant by solvent extraction, wherein the solvent can be a mixture of ethanol solution an...
Embodiment 2
[0055] Coumarin 6 was used as a fat-soluble model drug to study the effect of mSHS on Coumarin 6 drug loading and transdermal administration.
[0056] ①Drug loading process: Dichloromethane was used as a solvent to prepare Coumarin 6 solutions with different concentrations; weigh 10 mg mSHS and place it in a centrifuge tube, add 600 μl of Coumarin 6 with different concentrations, rotate and shake for 12 hours, and then mix mSHS and Coumarin 6 The mixed solution was centrifuged to remove all the supernatant drug solution, and the drug-loaded mSHS was placed in a fume hood to dry naturally to prepare the mSHS loaded with Coumarin 6 (mSHS@Coumarin 6). See Figure 4 .
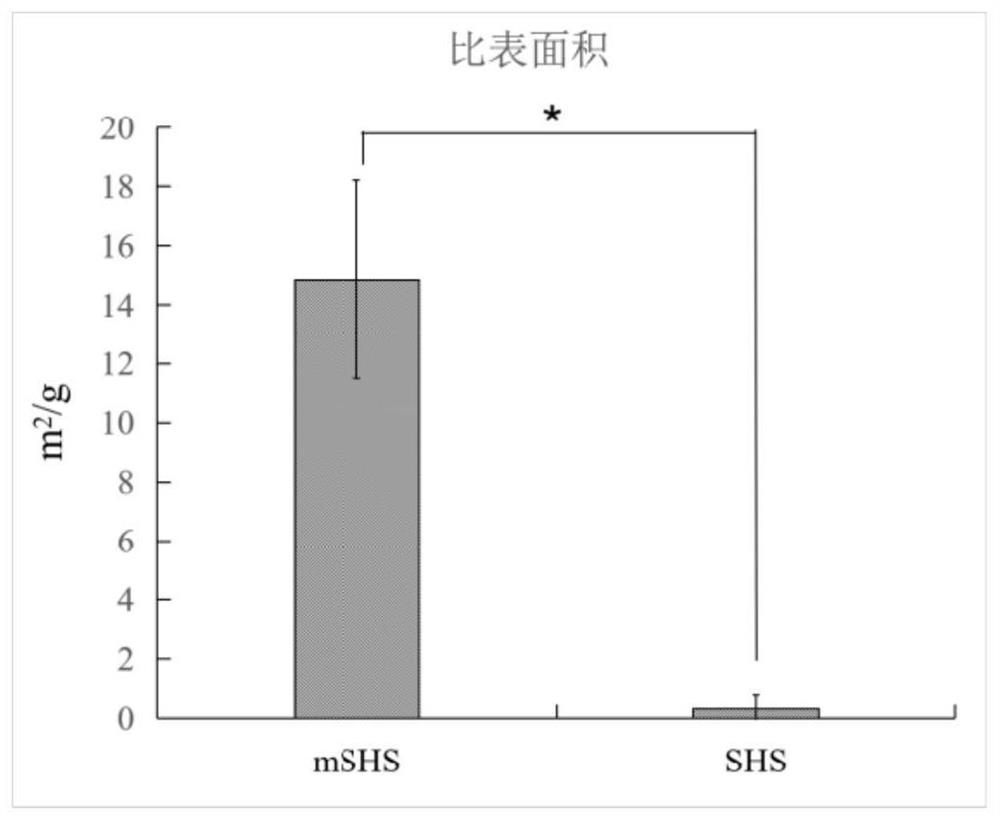
[0057] Put mSHS@Coumarin 6 in a certain amount of ethanol solution, place it on a shaker for 12h (28°C, 200rpm), and then calculate the drug loading corresponding to mSHS according to the content of Coumarin 6 in the ethanol solution. See the result Figure 7 .
[0058] Quantitative results of drug loading
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com