Antiviral ursolic acid derivative and preparation method thereof
A technology of viral ursolic acid and derivatives, applied in the directions of antiviral agents, steroids, organic chemistry, etc., can solve the problems of decreased yield of main products, unsuitable industrialization development, difficulty in solvent recovery, etc., and achieves improved solubility, Improved bioavailability, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
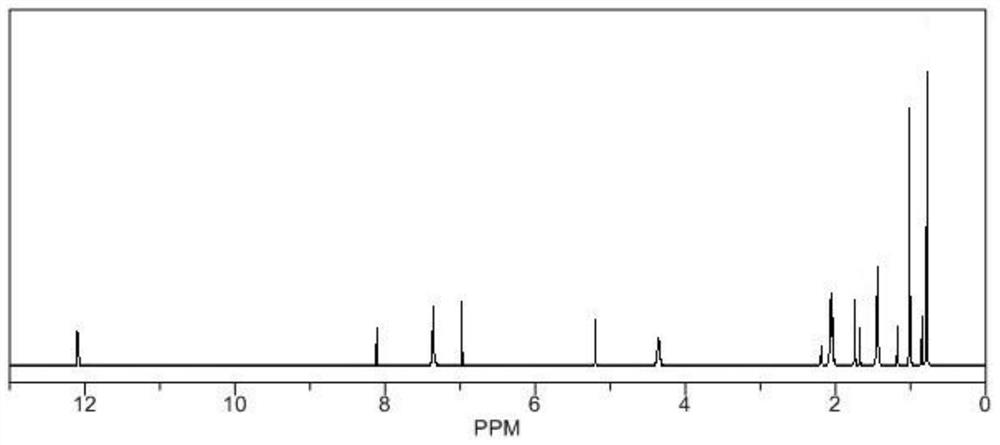
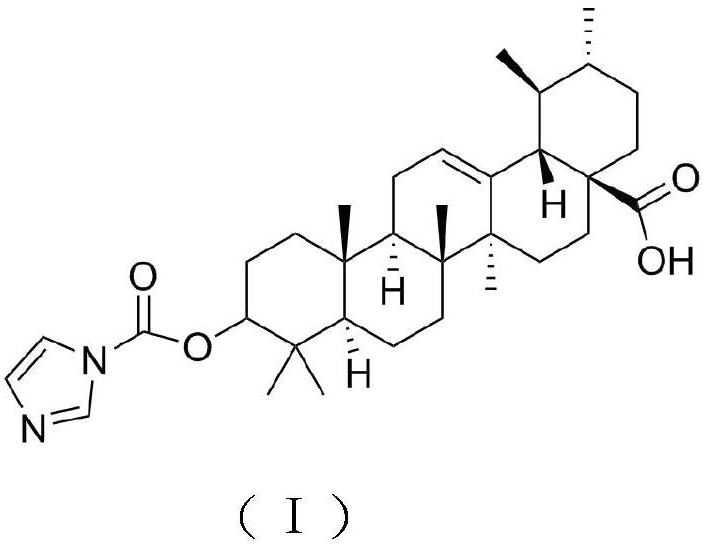
[0018] Weigh 20g of ursolic acid, add it into a 100mL round-bottomed flask, add 50mL of pyridine, stir well to dissolve, add 4g of N,N'-carbonyldiimidazole in batches, reflux at a temperature of 8-10°C for 4-6h, and detect by TLC After the reaction is completed, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, add 300-400 mesh silica gel to the filtrate, mix and dry, and elute by silica gel column chromatography (elution Agent: petroleum ether / ethyl acetate=3:1), the eluent was collected and concentrated, the concentrated solution was extracted with ethyl acetate, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 77.43%.
Embodiment 2
[0020] Weigh 20g of ursolic acid, add it to a 100mL round-bottomed flask, add 50mL of pyridine, stir well to dissolve, add 6g of N,N'-carbonyldiimidazole in batches, reflux at a temperature of 8-10°C for 4-6 hours, and detect by TLC After the reaction is completed, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, add 300-400 mesh silica gel to the filtrate, mix and dry, and elute by silica gel column chromatography (elution Agent: petroleum ether / ethyl acetate=3:1) The eluate was collected and concentrated, and the concentrated solution was extracted with ethyl acetate, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 78.69%.
Embodiment 3
[0022] Weigh 20g of ursolic acid, add it to a 100mL round-bottomed flask, add 50mL of pyridine, fully stir to dissolve, add 8g of N,N'-carbonyldiimidazole in batches, reflux at a temperature of 8-10°C for 4-6h, and detect by TLC After the reaction is completed, stop the reaction, concentrate under reduced pressure at 60°C to recover the solvent, then add absolute ethanol, stir to dissolve, filter, add 300-400 mesh silica gel to the filtrate, mix and dry, and elute by silica gel column chromatography (elution Agent: petroleum ether / ethyl acetate=3:1), the eluate was collected and concentrated, and the concentrated solution was extracted with ethyl acetate, crystallized, filtered, and vacuum-dried to obtain the target product with a yield of 79.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com