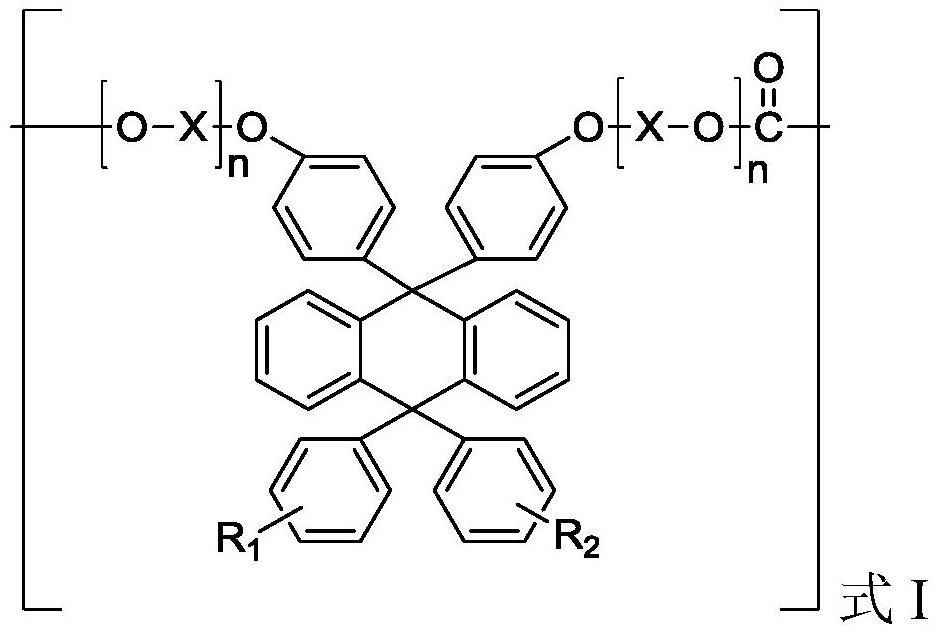
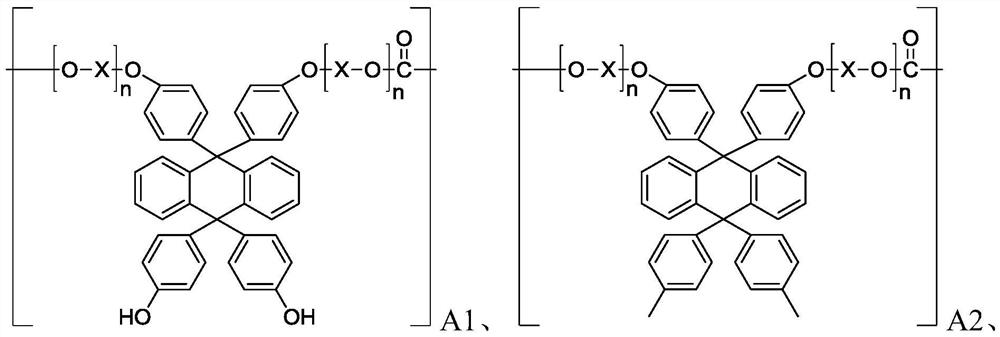
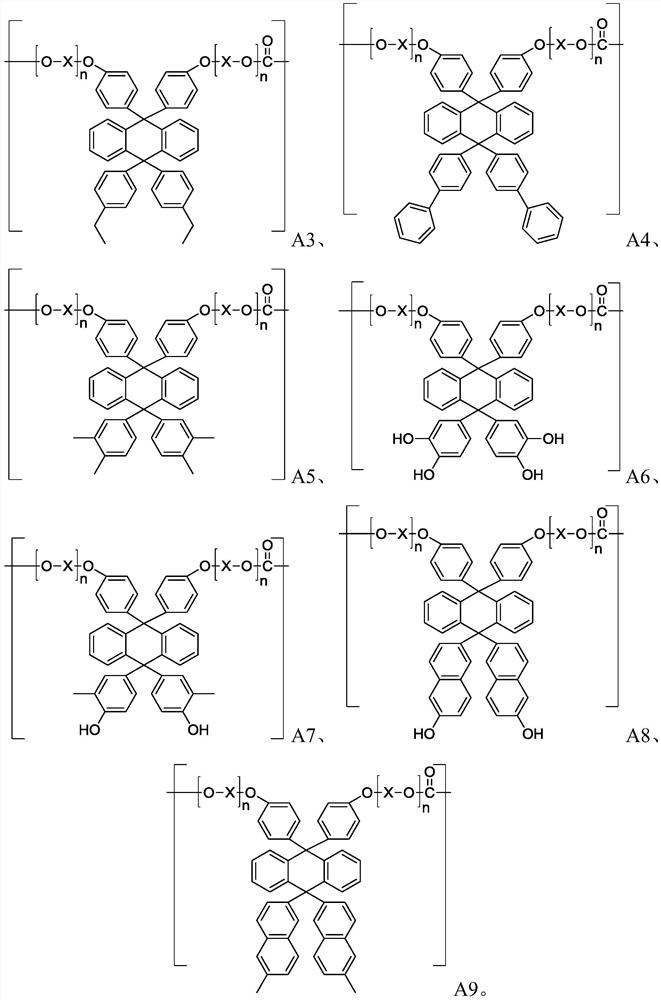
Polycarbonate resin, preparation method and formed optical component
A polycarbonate resin, polycarbonate technology, used in the field of preparation, optical components, polycarbonate resin, can solve problems such as inability to meet application requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Prepare dihydroxy compound Q1 according to the following synthetic route:
[0127]
[0128] (1) Synthesis of 10,10-bis(4-hydroxyethoxyphenyl)-9-hydrogen-anthracene
[0129] A 1L three-necked flask was placed in a constant temperature oil bath, and 194g of anthrone and 260g of phenoxyethanol were added for condensation reaction (the molar ratio was 1:2.1). The catalyst was 10g of concentrated sulfuric acid, and the auxiliary agent was 2.12g of mercaptopropionic acid. The temperature is 40°C, after stirring for 4 hours, the progress of the reaction is confirmed by HPLC, and the reaction is terminated when the residual amount of anthrone is below 0.1%.
[0130] Add 200g of methanol to the obtained reaction solution, ultrasonically dissolve, then add 200g of deionized water, a large amount of solids are precipitated, continue to stir at this temperature for 2h, cool down and suction filter to obtain a solid crude product; add the crude product to 200g of isopropanol In ...
Embodiment 2
[0138] [Preparation Example 2] (synthesis of dihydroxy compound Q2)
[0139] The reaction conditions are basically the same as those in Preparatory Example 1, except that the phenol in step (3) is changed to biphenyl to prepare the target product Q2, and its NMR hydrogen spectrum test data is as follows:
[0140] 1 H NMR (400MHz, CDCl 3 )δ7.52(d, J=8Hz, 4H), 7.51(d, J=8Hz, 4H), 7.41(d, J=8Hz, 2H), 7.33(d, J=8Hz, 4H), 7.31(d ,J=8Hz,4H),7.29(d,J=8Hz,4H),7.19(d,J=8Hz,4H),7.12(d,J=8Hz,4H),6.87(d,J=8Hz,4H ), 4.33(t, J=8Hz, 4H), 3.69(t, J=10Hz, 4H), 3.65(s, 2H).
Embodiment 3
[0141] [Preparation Example 3] (synthesis of dihydroxy compound Q3)
[0142] Reaction condition is substantially the same as preparation example 1, and difference is: the phenol in the step (3) is changed into o-cresol, prepares target product Q3, and its NMR hydrogen spectrum test data is as follows:
[0143] 1 H NMR (400MHz, CDCl 3 )δ7.31(d, J=8Hz, 4H), 7.19(d, J=8Hz, 4H), 7.12(d, J=8Hz, 4H), 6.93(s, 2H), 6.87(d, J=8Hz ,6H),6.71(d,J=8Hz,2H),5.35(s,2H),4.33(t,J=8Hz,4H),3.69(t,J=10Hz,4H),3.65(s,2H) ,2.15(s,6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com