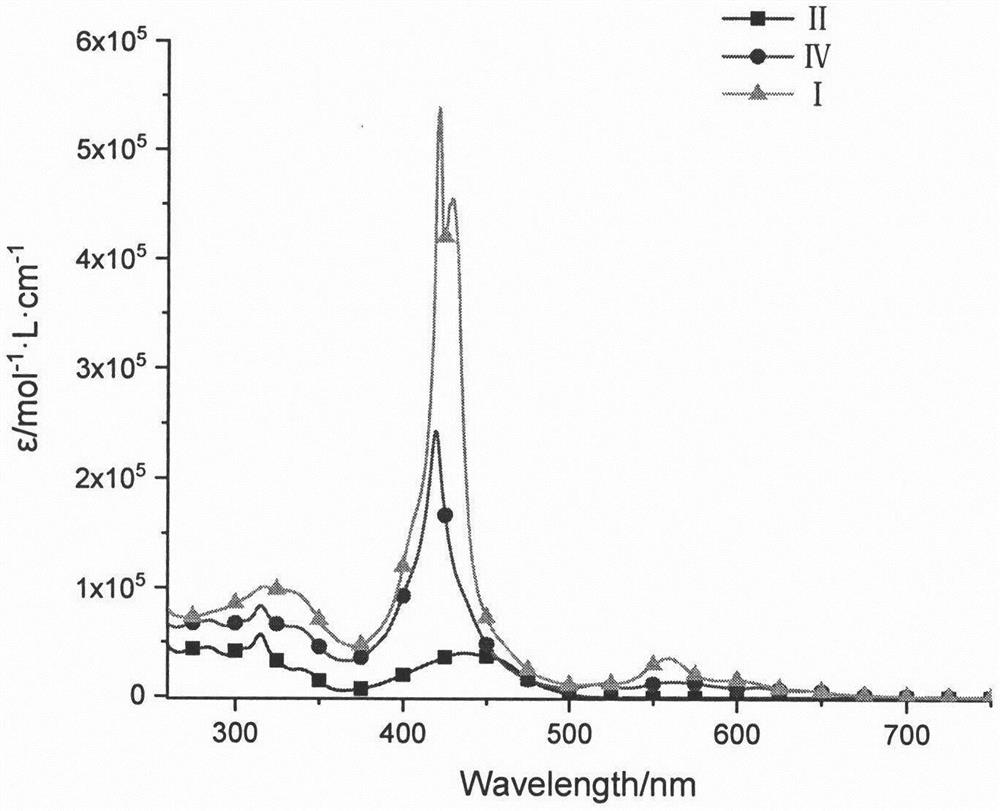
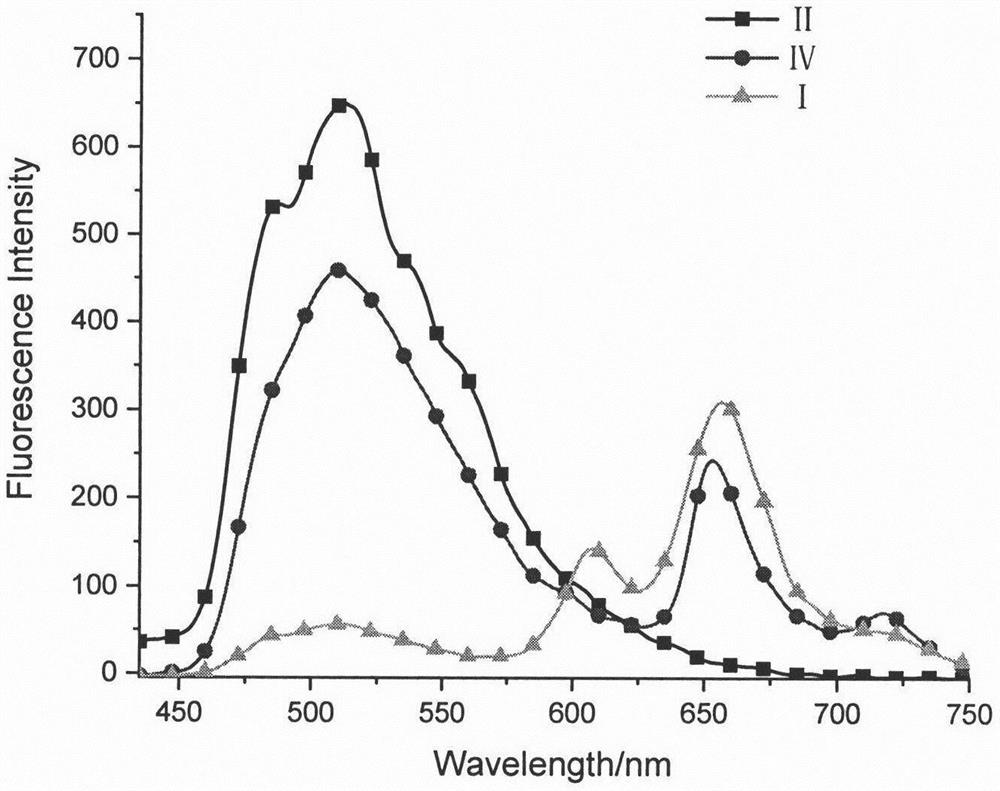
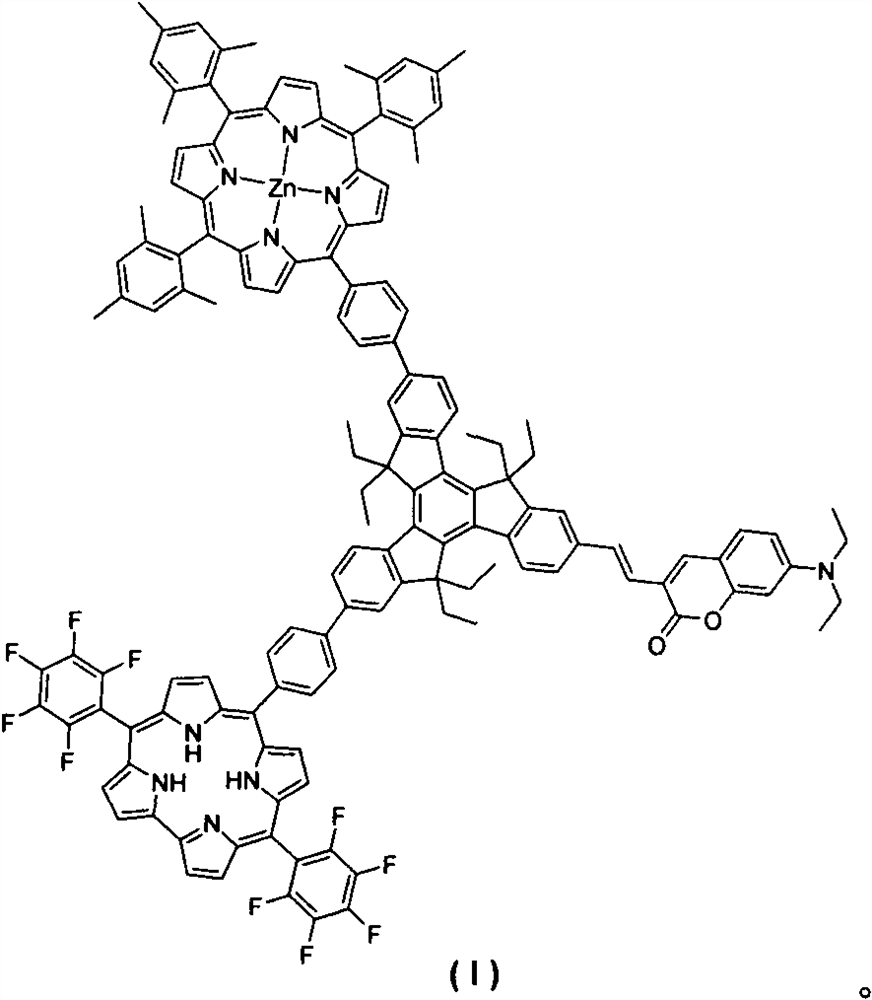
Synthesis and preparation method of tripolyindenyl coumarin-corrole-porphyrin quaternary system star-shaped compound
A technology of triindenyl-based triindenyl derivatives, which is applied in the field of preparation of star compounds based on triindenyl quaternary system, can solve the problems of complex reaction, many synthesis steps, and low yield, and achieve the goal of reaction The effect of good selectivity, simple synthetic route and simple separation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2,7-bisbromo-12-coumarinyltriindene derivative (II) (681.8mg, 0.75mmol), meso-phenylboronic ester corrole derivative (III) (582.4mg, 0.7mmol ), anhydrous potassium carbonate (1.04g, 7.5mmol) were dissolved in 30mL of tetrahydrofuran, and 3mL of water and 3mL of methanol were added thereto, and tetrakis(triphenylphosphine)palladium (58mg, 0.05mmol) was rapidly added thereto under argon protection , 65 ° C reaction 10h. After the reaction was completed, the solvent was spin-dried, dissolved in dichloromethane, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, evaporated under reduced pressure to dry the solvent, and dichloromethane-petroleum ether (v:v=1:1) The eluent was separated and purified by silica gel column chromatography to obtain the tripolyindenylcoumarin-corrole intermediate (IV) with a yield of 38%. 1 H NMR (CDCl 3 , 600MHz, ppm) δ9.14(d, J=3.6Hz, 2H), 8.87-8.80(m, 2H), 8.77-8.72(m, 2H), 8.59(s, 2H), 8.53-8.41(m, 1H), ...
Embodiment 2
[0026] Dissolve tripolyindenylcoumarin-corrole intermediate (IV), meso-phenyl borate zinc porphyrin derivative (V) (279mg, 0.3mmol), anhydrous potassium carbonate (345mg, 2.5mmol) In 20mL of tetrahydrofuran, 2mL of water and 2mL of methanol were added thereto, tetrakis(triphenylphosphine)palladium (12mg, 0.01mmol) was quickly added thereto under the protection of argon, and reacted at 65°C for 10h. After the reaction was completed, the solvent was spin-dried, extracted with dichloromethane, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, evaporated under reduced pressure to dry the solvent, and dichloromethane-petroleum ether (v:v=1:1) The eluent was separated and purified by silica gel column chromatography to obtain 113 mg of star compound (I) of ternary indenylcoumarin-corrole-porphyrin quaternary system, with a yield of 20%. 1 H NMR (CDCl 3 , 600MHz, ppm) δ9.15(s, 1H), 9.02(d, J=2.4Hz, 2H), 8.92-8.78(m, 5H), 8.76(m, 4H), 8.62(d, J=1...
Embodiment 3
[0028]2,7-bisbromo-12-coumarinyltriindene derivative (II) (681.8mg, 0.75mmol), meso-phenyl borate corrole derivative (III) (582.4mg, 0.7mmol ), anhydrous potassium carbonate (1.04g, 7.5mmol) were dissolved in 30mL of tetrahydrofuran, and 3mL of water and 3mL of methanol were added thereto, and tetrakis(triphenylphosphine) palladium (35mg, 0.03mmol) was rapidly added thereto under argon protection , Reaction at 75°C for 8h. After the reaction was completed, the solvent was spin-dried, dissolved in dichloromethane, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, evaporated under reduced pressure to dry the solvent, and dichloromethane-petroleum ether (v:v=1:1) The eluent was separated and purified by silica gel column chromatography to obtain the tripolyindenylcoumarin-corrole intermediate (IV) with a yield of 31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com