Refining method of azacitidine with high purity and low residual solvent content
A technology of azacitidine and refining methods, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., and can solve problems such as low refining yield, non-conformity of product quality, and non-conformity of refined products to quality standards , to achieve the effect of high refining yield, low solvent residue and stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] According to the preparation process of the present invention, the obtained samples are vacuum-dried, the drying temperature is generally 50°C-90°C, preferably 60°C-80°C; the drying time is 12-24 hours.
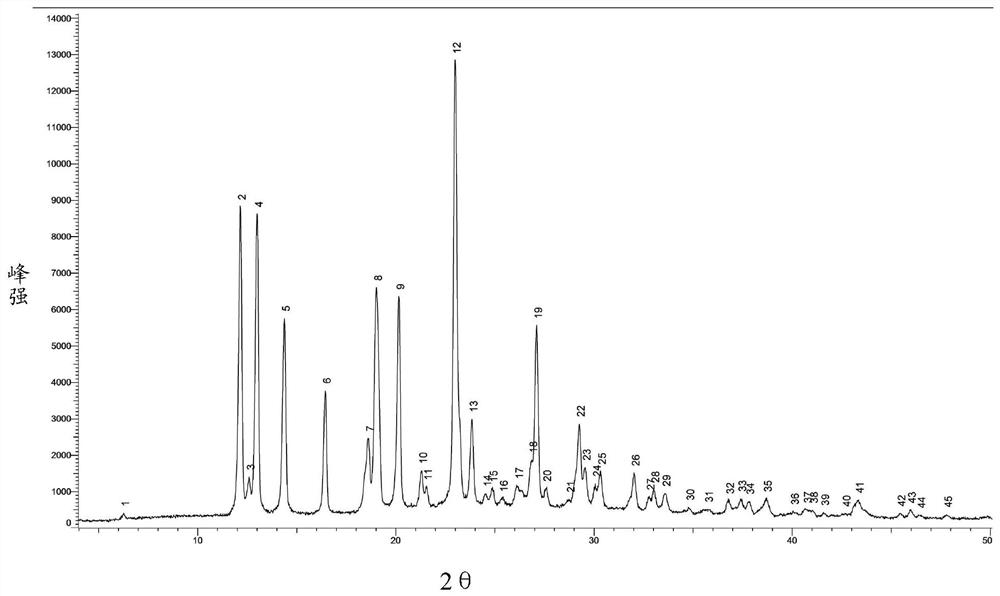
[0055] The azacitidine crystal form obtained according to the preparation process of the present invention is crystal form I, which can be used for the treatment of myelodysplastic syndrome, leukemia and other diseases.
[0056] The present invention and its beneficial technical effects will be described in detail below in conjunction with the accompanying drawings and various specific embodiments.
Embodiment 1
[0064] Add 300ml DMSO to a 2L reaction flask, continue to add 50g of crude azacitidine, under nitrogen protection, heat the solution to 40°C, stir to dissolve; slowly add 900ml ethyl acetate, slowly cool down to 20°C, stir for 2h; filter , to obtain a primary refined product with a wet weight of 52.2g. The weight loss on drying was measured, and the dry weight was 47.7g, and the yield was 95.4%.
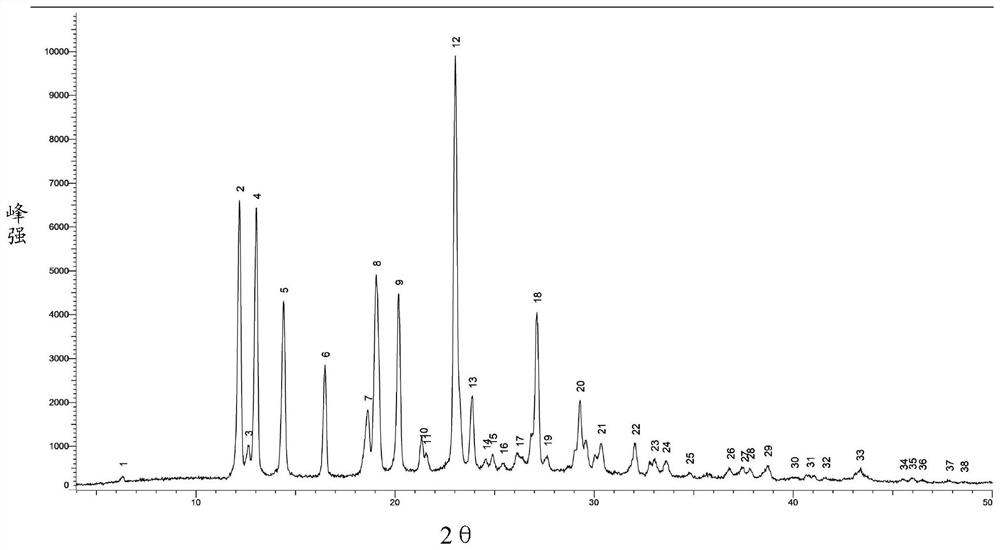
[0065] Add 280ml of DMSO into a 2L reaction flask, continue to add a refined product (wet weight 55.2g), under nitrogen protection, heat to 35°C, stir to dissolve; slowly add 860ml of methanol, slowly cool down to 15°C, stir for 3 hours, filter The obtained filter cake was beaten three times with 280ml methanol respectively, filtered, dried under reduced pressure at 80°C (controlling vacuum degree ≤-0.08MPa), and dried for 12h to obtain 46.7g, with a refined total yield of 90.4%. Measured product purity 99.77%, monoacetyl impurity (formula II) 0.04%, DMSO solvent residual 1120ppm, X...
Embodiment 2
[0067] Add 200ml DMSO into a 2L reaction flask, continue to add 50g of crude azacitidine, under nitrogen protection, heat the solution to 45°C, stir to dissolve; slowly add 800ml of isopropyl acetate, slowly cool down to 15-20°C, stir 2h; filter to obtain a primary refined product with a wet weight of 53.0g. The weight loss on drying was measured, and the dry weight was 45.1g, and the yield was 90.2%.
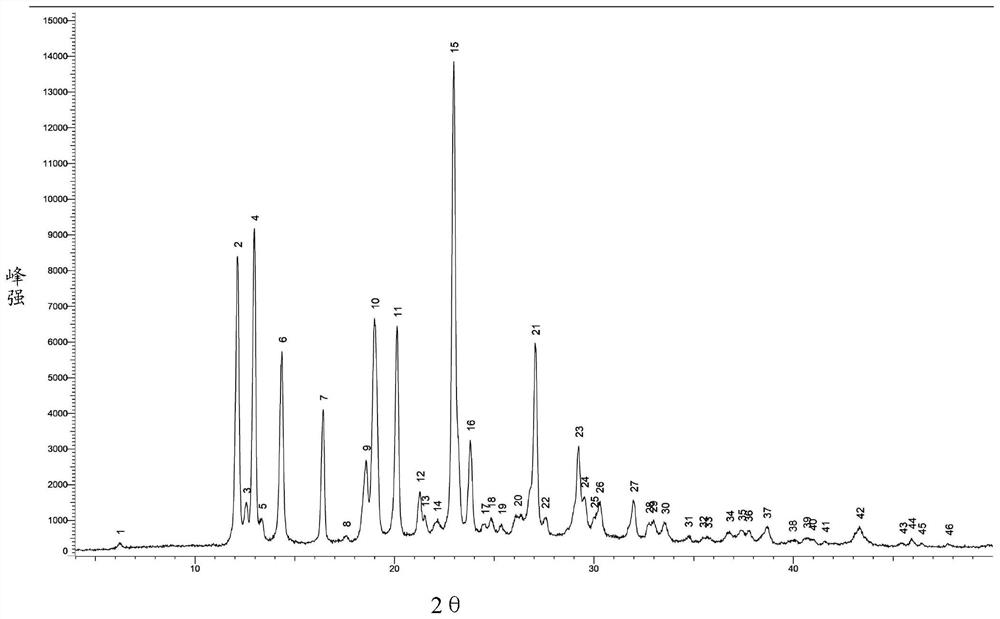
[0068] Add 170ml DMSO to a 2L reaction flask, continue to add a refined product (wet weight 53.0g), under nitrogen protection, heat to 45°C, stir to dissolve; slowly add 680ml of isopropanol, slowly cool down to 15-20°C, stir After 3 hours, filter; the obtained filter cake was beaten three times with 170ml of isopropanol, filtered, dried under reduced pressure at 75°C (controlling vacuum degree ≤-0.08MPa), and dried for 18h to obtain 43.6g, with a refined total yield of 87.1%. Measure product purity 99.61%, monoacetyl impurity (formula II) 0.08%, DMSO solvent residual 2700ppm,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com