SARS-CoV-2 polypeptide vaccine using papillomavirus (PV) virus-like particle (VLP) presentation antigen
A virus-like particle, coronavirus technology, applied in the field of novel coronavirus polypeptide vaccines, which can solve the problems of no antiviral drugs or regulatory approval of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: Design and synthesis of codon-optimized genes
[0117] Referring to the codon preference of Escherichia coli for gene transcription, the coding sequence of the SARS-CoV-2 spike protein epitope polypeptide was optimized using bioinformatics software to adapt to high-efficiency expression in Escherichia coli, and then the coding sequences were cloned into the expression papilloma In the recombinant plasmid of virus (PV) L1 protein, the insertion site can be DE loop, BC loop, FG loop or HI loop. Afterwards, the L1 recombinant plasmid of the chimeric SARS-CoV-2 spike protein epitope polypeptide coding sequence was transformed into Escherichia coli for expression.
[0118] Specifically: first use the online bioinformatics software CodonAdaptationTool (JCAT) (http: / / www.jcat.de / ) to optimize the coding SARS-CoV-2 spike protein epitope polypeptide SEQ ID NO: 1, SEQ ID NO: 2 and the codon sequence of SEQID NO:3. The codon-optimized gene sequence was outsourced and ...
Embodiment 2
[0120] Embodiment 2: Expression and purification of HPV L1 protein of chimeric SARS-CoV-2 spike protein epitope polypeptide
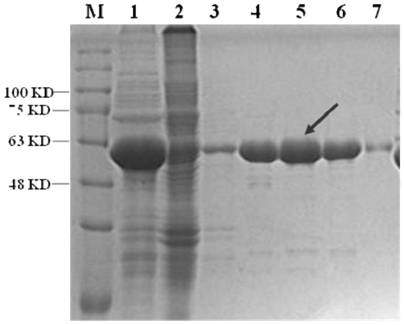
[0121] Identify the correct recombinant plasmid, and transform the recombinant plasmid of the chimeric SARS-CoV-2 spike protein epitope polypeptide into the Escherichia coli expression strain BL21. Pick a single clone and inoculate it into LB liquid medium containing ampicillin resistance, culture overnight at 37°C, inoculate the culture in fresh LB medium containing ampicillin resistance at a volume ratio of 1:100, and culture it with shaking at 37°C until When OD600nm is about 0.8, add IPTG with a final concentration of 1 mM, induce expression at 37°C for 6 hours, or induce overnight at 25°C. The cells induced to express the fusion protein at 37°C were collected by centrifugation. Cells were lysed with a high-pressure disruptor and centrifuged at high speed (12,000g) for 1 hour. The supernatant was collected and 20% ammonium sulfate was added for pri...
Embodiment 3
[0122] Example 3: Identification of HPV L1-VLP of Chimeric SARS-CoV-2 Spike Protein Epitope Polypeptide
[0123] After completing all the purification steps, the HPV L1-VLP sample of the obtained chimeric SARS-CoV-2 spike protein epitope polypeptide was subjected to dynamic light scattering and electron microscope observation to identify its particle size and shape.
[0124] Specifically, after the HPV L1-VLP solution of the chimeric SARS-CoV-2 spike protein epitope polypeptide obtained after purification was filtered through a 0.22 μm filter membrane, the particle size distribution and particle uniformity of the particles were analyzed using a Malvern dynamic light scattering instrument. Once the situation. image 3 Shown is the HPV16 VLP particle size detection result of the chimeric novel coronavirus spike protein epitope polypeptide.
[0125] Z average particle size (d.nm): 51.48; PdI: 0.012; intercept: 0.961; product quality: good.
[0126] Table 1: Intensity-force-diam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com