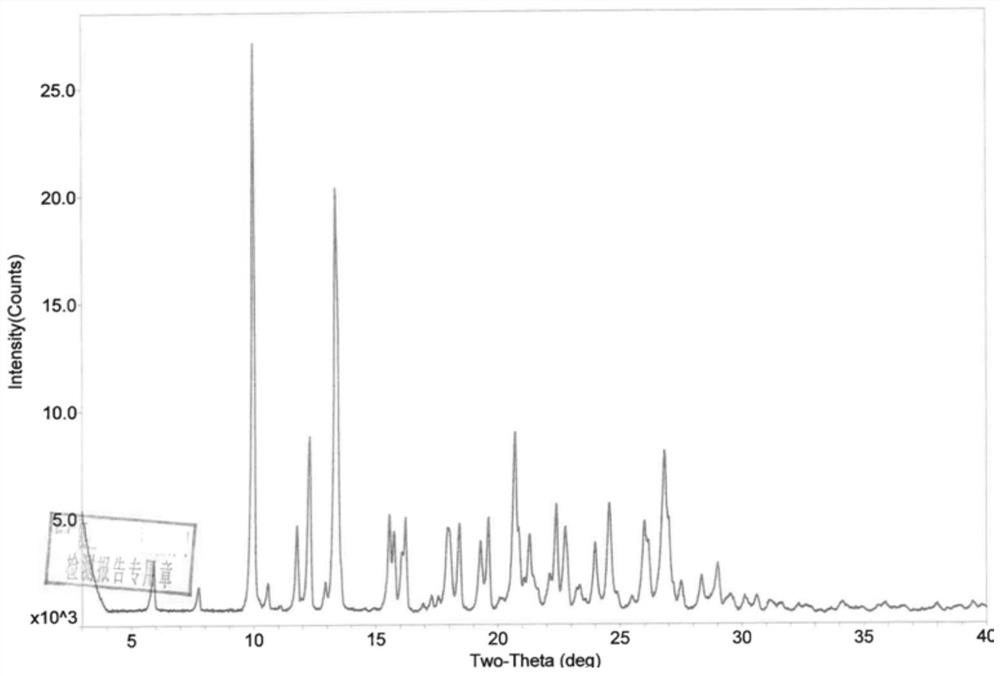
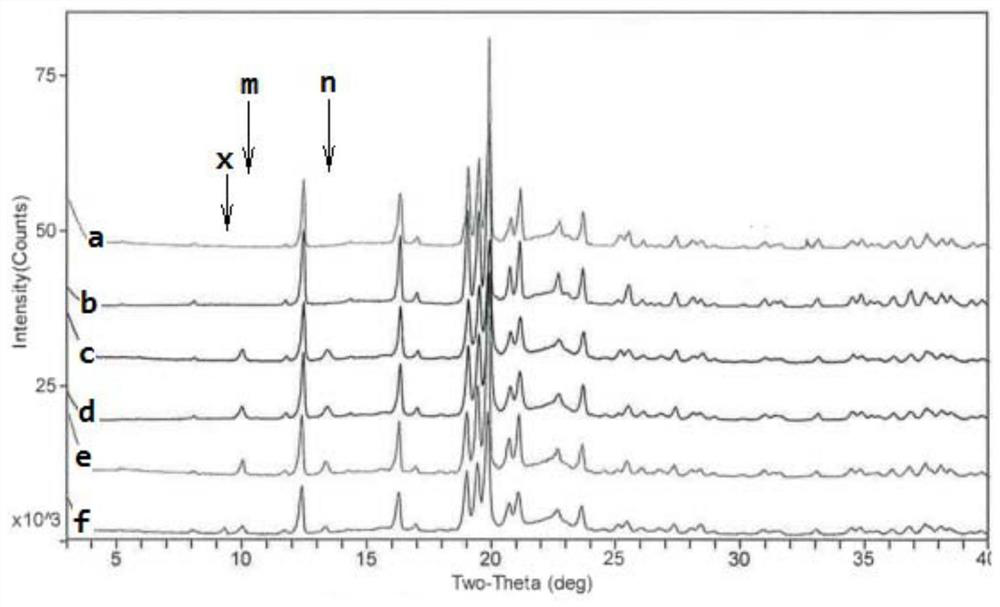
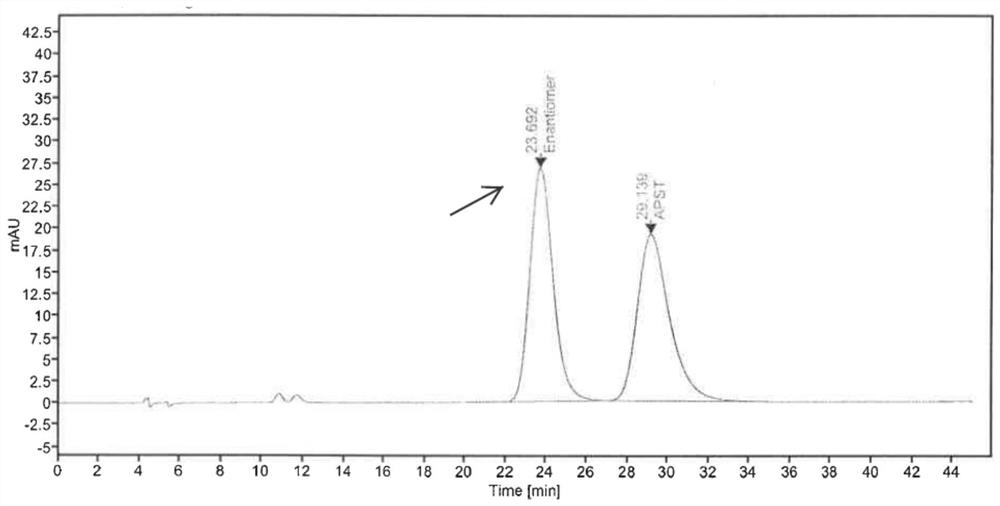
Phosphodiesterase-4 inhibitor apremilast composition and quality detection method
A technology of Apos and content, applied in the field of medicine, can solve the problem that the exact mechanism cannot be finally confirmed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0122] Preparation example 1, prepare the plain tablet of Apremilast
[0123] Prescription: 10 parts by weight of Apremilast, 60 parts by weight of lactose monohydrate, 26.25 parts by weight of microcrystalline cellulose, 3 parts by weight of croscarmellose sodium (internal addition: external addition = 2:1), stearin Magnesium acid 0.75 parts by weight.
[0124] Preparation method:
[0125] (i) Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium are mixed uniformly, and the granules are prepared by dry method using pressing roller equipment;
[0126] (ii) add surplus croscarmellose sodium to the granule obtained in step (i), mix uniformly, then add magnesium stearate and mix uniformly;
[0127] (iii) Compressing the mixed granules obtained in step (ii) on a tablet machine to obtain tablets in the form of plain tablets.
[0128] The term "internal addition" is added before the granules are made to prepare tablets, that is, the croscarmellose sodium p...
preparation example 2
[0129] Preparation example 2, prepare the plain tablet of Apremilast
[0130] Prescription: 10 parts by weight of Apremilast, 62 parts by weight of lactose monohydrate, 22 parts by weight of microcrystalline cellulose, 3.5 parts by weight of croscarmellose sodium (internal addition: external addition = 2:1), stearin Magnesium acid 0.9 parts by weight.
[0131] Preparation method:
[0132] (i) Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium are mixed uniformly, and the granules are prepared by dry method using pressing roller equipment;
[0133] (ii) add surplus croscarmellose sodium to the granule obtained in step (i), mix uniformly, then add magnesium stearate and mix uniformly;
[0134] (iii) Compressing the mixed granules obtained in step (ii) on a tablet machine to obtain tablets in the form of plain tablets.
preparation example 3
[0135] Preparation example 3, prepare the plain tablet of Apremilast
[0136] Prescription: 10 parts by weight of Apremilast, 55 parts by weight of lactose monohydrate, 28 parts by weight of microcrystalline cellulose, 2.5 parts by weight of croscarmellose sodium (internal addition: external addition = 2:1), stearin Magnesium acid 0.6 parts by weight.
[0137] Preparation method:
[0138] (i) Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium are mixed uniformly, and the granules are prepared by dry method using pressing roller equipment;
[0139] (ii) add surplus croscarmellose sodium to the granule obtained in step (i), mix uniformly, then add magnesium stearate and mix uniformly;
[0140] (iii) Compressing the mixed granules obtained in step (ii) on a tablet machine to obtain tablets in the form of plain tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com