Pingyangmycin-containing nanoemulsion contrast therapeutic agent and preparation process thereof
A technology of Pingyangmycin and its preparation process, which is applied in the preparation of X-ray contrast agents, general/multifunctional contrast agents, preparations for in vivo tests, etc. It can solve the problems of life-threatening and high cancer mortality, and achieve the improvement of contrast effect , remarkable effect and reasonable structural design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
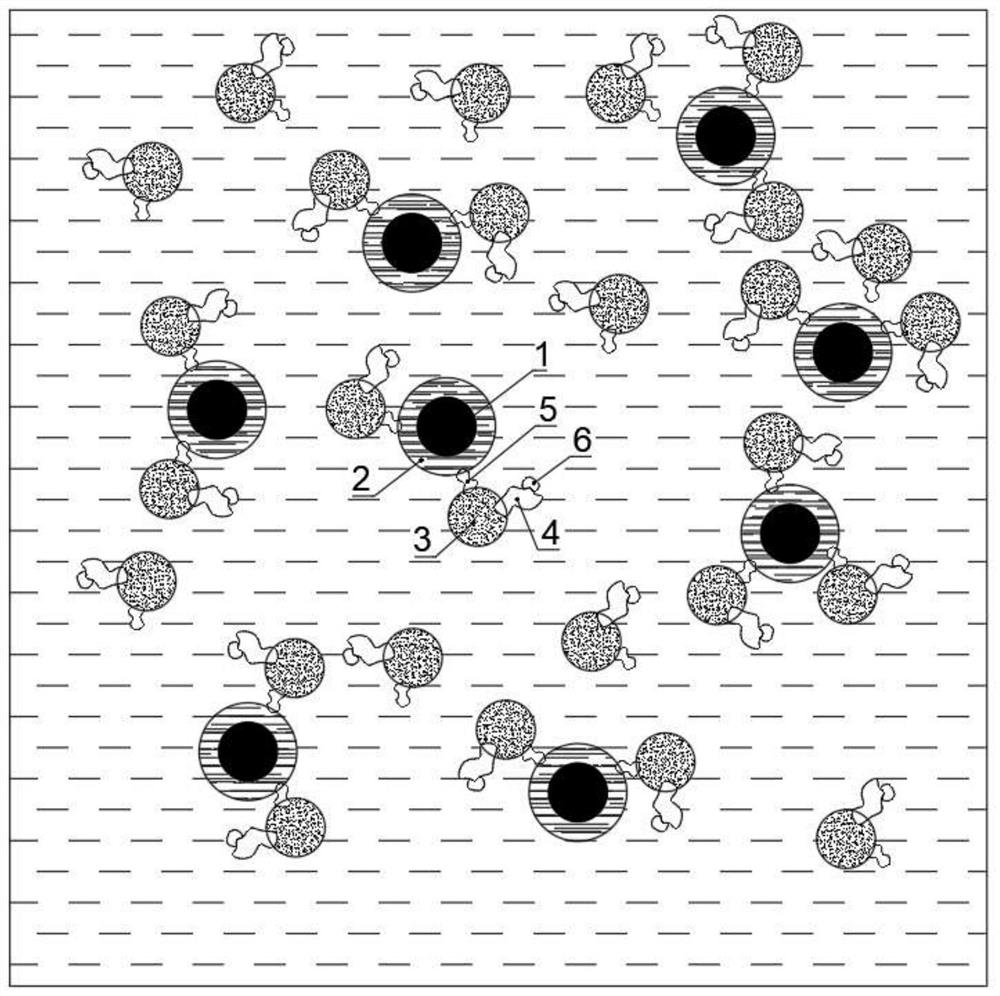
[0035]A nanoemulsion contrast treatment agent containing Pingyangmycin, comprising: 70 parts of superparamagnetic nanoparticles, 400 parts of iodized oil, 135 parts of medical compound gelatin, 1 part of Pingyangmycin, 15 parts of serum white egg white, 5 parts emulsifier.
[0036] Wherein, the superparamagnetic nanoparticles are Fe 2 o 3 Nanoparticles are obtained by evaporation method, and the diameter of the superparamagnetic nanoparticles is 3nm.
[0037] The Fe 2 o 3 Nanoparticles are encapsulated within the emulsified lipiodol emulsion droplets.
[0038] The medical composite gelatin comprises bone pharmaceutical gelatin and skin pharmaceutical gelatin with a mass ratio of 10:3.
[0039] The iodine content of the iodized oil is 100mg / ml.
[0040] The serum albumin is combined on the emulsion droplet made of the medical compound gelatin, and the pingyangmycin is combined on the serum albumin.
[0041] The emulsifier includes 50 parts of surfactant and 1 part of co-...
Embodiment 2
[0045] A nanoemulsion contrast treatment agent containing pingyangmycin, comprising: 80 parts of superparamagnetic nanoparticles, 450 parts of iodized oil, 155 parts of medical compound gelatin, 2 parts of pingyangmycin, 20 parts of serum white egg white, 8 parts emulsifier.
[0046] Wherein, the superparamagnetic nanoparticles are Fe 2 o 3 Nanoparticles, which are obtained by evaporation method, the diameter of the superparamagnetic nanoparticles is 7nm.
[0047] The Fe 2 o 3 Nanoparticles are encapsulated within the emulsified lipiodol emulsion droplets.
[0048] The medical composite gelatin comprises bone pharmaceutical gelatin and skin pharmaceutical gelatin with a mass ratio of 10:3.
[0049] The iodine content of described iodized oil is 125mg / ml.
[0050] The serum albumin is combined on the emulsion droplet made of the medical compound gelatin, and the pingyangmycin is combined on the serum albumin.
[0051] The emulsifier includes 55 parts of surfactant and 1....
Embodiment 3
[0055] A nanoemulsion contrast treatment agent containing pingyangmycin, comprising: 90 parts of superparamagnetic nanoparticles, 500 parts of iodized oil, 175 parts of medical compound gelatin, 3 parts of pingyangmycin, 25 parts of serum white egg white, 10 parts emulsifier.
[0056] Wherein, the superparamagnetic nanoparticles are Fe 2 o 3 Nanoparticles, which are obtained by evaporation method, the diameter of the superparamagnetic nanoparticles is 10nm.
[0057] The Fe 2 o 3 Nanoparticles are encapsulated within the emulsified lipiodol emulsion droplets.
[0058] The medical composite gelatin comprises bone pharmaceutical gelatin and skin pharmaceutical gelatin with a mass ratio of 10:3.
[0059] The iodine content of described iodized oil is 150mg / ml.
[0060] The serum albumin is combined on the emulsion droplet made of the medical compound gelatin, and the pingyangmycin is combined on the serum albumin.
[0061] The emulsifier includes 60 parts of surfactant and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com