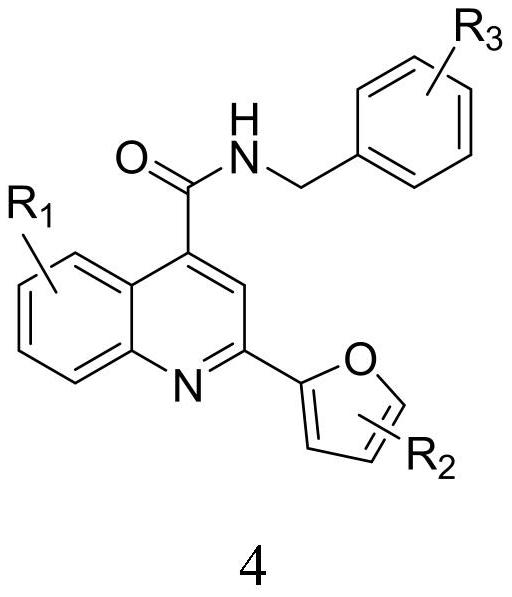
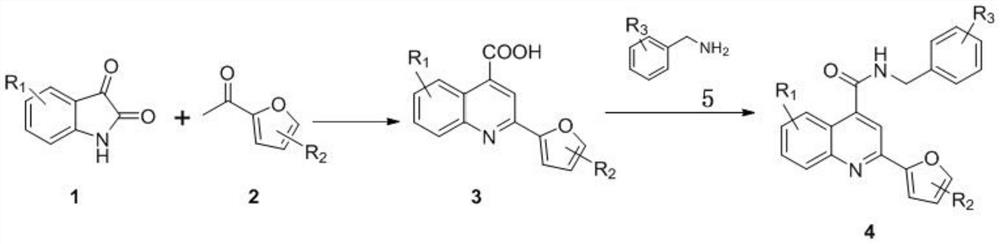
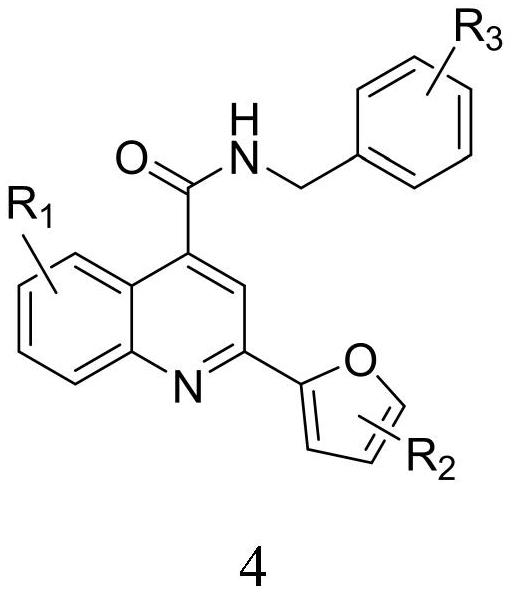
2-furanyl-quinoline-4-formamide compound and application thereof
A technology of carboxamides and compounds, which is applied in the field of 2-furan-quinoline-4-carboxamide compounds, can solve the problems of tumors, decreased activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of 2-(5-methylfuran-2-yl)quinoline-4-carboxylic acid (3a)
[0019]
[0020] 0.3g (2.0mmol) isatin (1a), 0.7g mass concentration is 33% KOH aqueous solution and 0.25g (2.0mmol) 5-methyl-2-acetylfuran (2a) are dissolved in 10mL ethanol, heating React at 80°C for 24 hours. After the reaction, concentrate under reduced pressure to remove the solvent to obtain a residue, add 10wt% acetic acid aqueous solution, adjust the pH to about 5, cool and crystallize, filter to obtain a solid crude product, wash with appropriate amount of ethanol and petroleum ether, and dry to obtain 0.31g of 3a compound (ie 2-(5-methylfuran-2-yl)quinoline-4-carboxylic acid), yield 60.4%, melting point: >250°C. 1 H NMR (500MHz, DMSO-d 6 )δ8.64-8.58(m,1H),8.14(s,1H),8.02(d,J=8.5Hz,1H),7.80-7.72(m,1H),7.62-7.54(m,1H),7.31 (d,J=3.3Hz,1H),6.39-6.34(m,1H),2.44(s,3H).
Embodiment 2
[0021] Example 2: Preparation of 6-fluoro-2-(5-methylfuran-2-yl)quinoline-4-carboxylic acid (3b)
[0022]
[0023] The preparation method of Example 2 was repeated in Example 1, except that "isatin (1a) was replaced by 5-fluoroisatin (1b) in an equivalent molar amount", and the rest of the operating steps were the same as in Example 1. Compound 3b was finally obtained with a yield of 87.5% and melting point: >250°C.
Embodiment 3
[0024] Example 3: Preparation of 8-fluoro-2-(5-methylfuran-2-yl)quinoline-4-carboxylic acid (3c)
[0025]
[0026] The preparation method of Example 3 was repeated in Example 1, except that "isatin (1a) was replaced by 7-fluoroisatin (1c) in an equivalent molar amount", and the rest of the operation steps were the same as in Example 1. Compound 3c was finally obtained with a yield of 62.4% and a melting point of >250°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com