Method for purifying pertussis fimbrillin
A fimbrial protein and pertussis technology, applied in the biological field, can solve the problems of many steps, high production cost, long time consumption, etc., and achieve good repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]Example 1. Fermentation culture and pretreatment of Bordetella pertussis
[0051]The pertussis strain was fermented and cultured, the culture supernatant and the bacterial pellet were harvested, and 10-15 times the volume of the precipitation (wet weight) of the 50 mM PB buffer containing 1 M sodium chloride at pH=8.0 was added to the bacterial pellet and stirred Evenly, leaching at 2~8℃ for 1-5 hours (preferably 1.5 hours). After centrifugation, the leaching supernatant and the leaching precipitate are collected, and the crude PRN solution is separated from the leaching precipitate, and passed through the Capto Adhere layer After the column is separated, the flow-through liquid is collected (for the specific method, please refer to our company's previous patent, patent number CN108570098A).
Embodiment 2
[0052]Example 2. Convective flow-through is firstly salted out with ammonium sulfate and then passed through a Capto Adhere column
[0053]1. Slowly add solid ammonium sulfate to the PRN chromatographic flow-through solution obtained in Example 1 at 2-8°C, so that the final concentration of ammonium sulfate reaches (mass volume ratio W / V) 15-25%, and stir slowly Dissolve completely after 1 hour,
[0054]2. Leave the solution to stand for 16-24 hours, centrifuge at 15000×g for 30 minutes, dissolve the precipitate with 50mM Tris-HCl buffer with a pH of 8.0-9.0, fully dissolve it and centrifuge again. The centrifugal conditions are the same as the above, and collect after centrifugation. Supernatant solution.
[0055]3. Adjust the pH and conductivity of the supernatant solution to 8.0-9.0 and 20-30mS / cm, respectively, and collect the flow-through after passing through the Capto Adhere column to obtain the crude fimbrin (Fim) pure liquid;
Embodiment 3
[0056]Example 3. The flow-through liquid was first collected through a Capto Adhere chromatography column and the flow-through was added with ammonium sulfate salting out
[0057]1. Adjust the pH and conductivity of the flow-through fluid collected in Example 1 to 8.0-9.0 and 20-30 mS / cm, respectively, and collect the flow-through fluid after passing through a Capto Adhere column;
[0058]2. Under the condition of 2-8℃, slowly add ammonium sulfate solid to make the final concentration of ammonium sulfate reach (mass volume ratio W / V) 15-25%, stir slowly for 1 hour and then dissolve completely.
[0059]3. Leave the solution to stand for 16-24 hours, centrifuge at 15000×g for 30 minutes, dissolve the precipitate with 50mM Tris-HCl buffer of pH 8.0-9.0, and centrifuge again after fully dissolving. The centrifugal conditions are the same as the above, and collect after centrifugation. The supernatant solution is used to obtain a crude fimbrin (Fim) pure liquid.
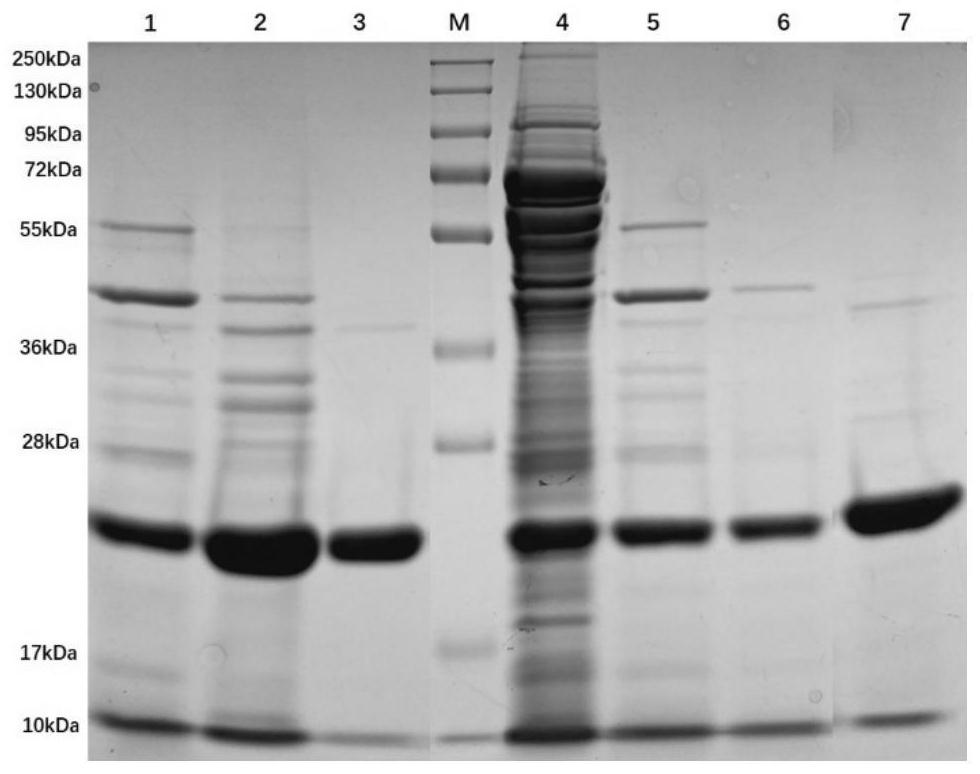
[0060]The SDS-PAGE diagrams of samp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com