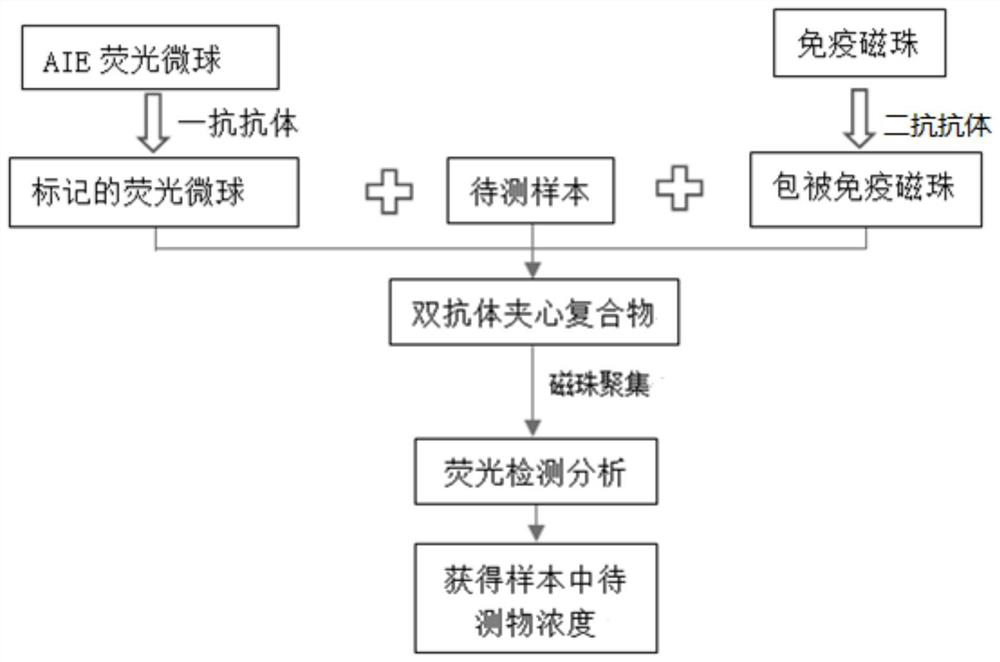
Method for detecting aggregation-induced emission combined with immunomagnetic beads and kit thereof
A technology of aggregation-induced luminescence and immunomagnetic beads, which is applied in the direction of analysis, measurement device, fluorescence/phosphorescence, etc. through chemical reaction of materials, which can solve the problems of many detection steps, difficulty in multi-index joint inspection, and environmental pollution by cleaning waste liquid. , to shorten the detection time, improve the detection sensitivity and accuracy, and omit the washing step.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
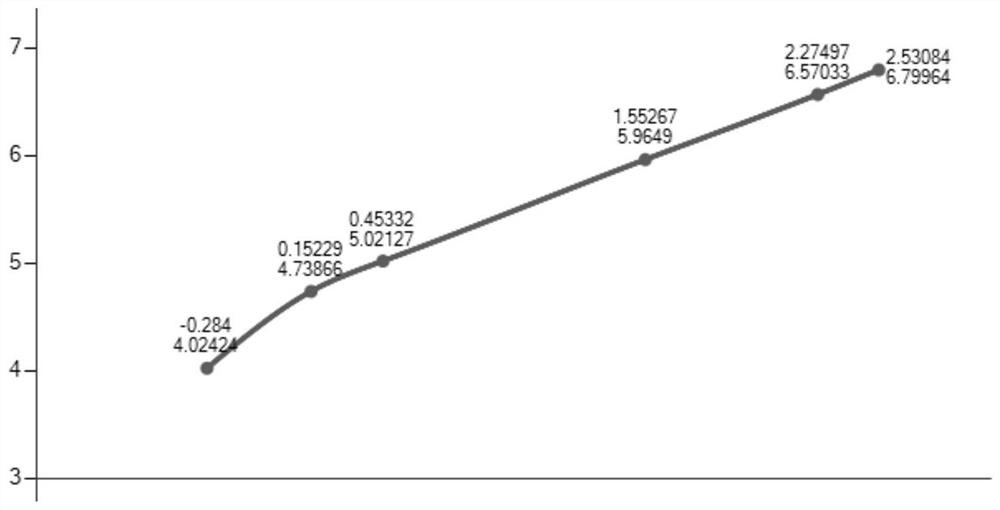
[0037] Example 1 Quantitative detection of C-reactive protein (CRP)
[0038] This example takes TPE-COOH aggregation-induced fluorescent microspheres as an example, and the specific operations are as follows:
[0039] The C-reactive protein standard was diluted to 0 ng / ml, 0.52 ng / ml, 1.42 ng / ml, 2.84 ng / ml, 35.7 ng / ml, 188.35 ng / ml, 339.5 ng / ml.
[0040] The labeled value of the sample to be tested is 22.35ng / ml.
[0041] 1. Aggregation-induced fluorescent microspheres label antigens or antibodies to obtain fluorescent markers
[0042] 1.1 Labeled antibody / antigen pretreatment: Take the C-reactive protein primary antibody and add it to a 30KD ultrafiltration centrifuge tube, and centrifuge at 10000r / min at 4°C for 9min to 10min. Repeat washing with binding buffer 3 to 5 times, and concentrate to a certain concentration (such as 1 mg / ml). The binding buffer is 0.05M Tris-HCl buffer at pH 8.0-9.0.
[0043] 1.2 Aggregation-induced fluorescent microspheres labeled with anti...
Embodiment 2
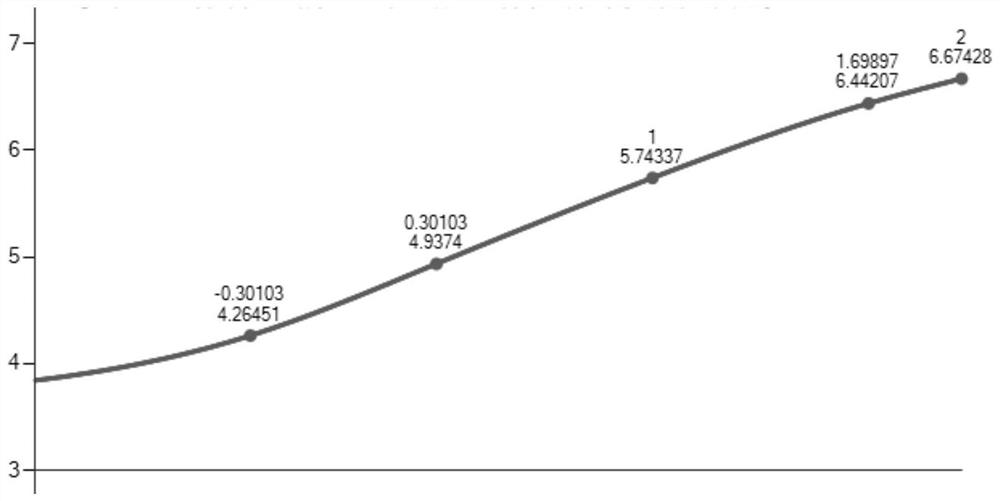
[0054] Example 2 Quantitative detection of procalcitonin (PCT)
[0055] This example takes TPE-CHO aggregation-induced fluorescent microspheres as an example, and the specific operations are as follows:
[0056] The procalcitonin standard was diluted to 0 ng / ml, 0.02 ng / ml, 0.5 ng / ml, 2 ng / ml, 10 ng / ml, 50 ng / ml, 100 ng / ml.
[0057] The labeled value of the sample to be tested is 0.452ng / ml.
[0058] 1. Aggregation-induced fluorescent microspheres label antibodies to obtain fluorescent markers
[0059] 1.1 Labeled antibody pretreatment: Take procalcitonin primary antibody and add it into a 30KD ultrafiltration centrifuge tube, and centrifuge at 10000r / min at 4°C for 9min to 10min. Repeat washing with binding buffer 3 to 5 times, and concentrate to a certain concentration (such as 1 mg / ml). The binding buffer is 0.05M Tris-HCl buffer at pH 8.0-9.0.
[0060] 1.2 Aggregation-induced fluorescent microsphere-labeled antibody: Wash aggregation-induced fluorescent microspheres ...
Embodiment 3
[0071] Example 3 Dual-label quantitative detection of C-reactive protein (CRP) and serum amyloid A (SAA)
[0072] In order to achieve the purpose of detection, this embodiment adopts TPE-2SO4 with similar wavelengths of excitation light and different wavelengths of emission light using aggregation-induced fluorescent materials. 3 Na + and TPE-COOH two aggregation-induced fluorescent microspheres as examples to label C-reactive protein (CRP) and serum amyloid A (SAA) respectively, the specific operations are as follows:
[0073] The C-reactive protein standard was diluted to 0 ng / ml, 0.52 ng / ml, 1.42 ng / ml, 2.84 ng / ml, 35.7 ng / ml, 188.35 ng / ml, 339.5 ng / ml.
[0074] The serum amyloid standard was diluted to 0 ng / ml, 0.93 ng / ml, 3.57 ng / ml, 15.45 ng / ml, 73.39 ng / ml, 140.19 ng / ml, 282.82 ng / ml.
[0075] The sample to be tested contains both C-reactive protein and serum amyloid, and the marked value of C-reactive protein is 10.05 ng / ml, and the marked value of serum amyloid is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com