Immune cell storage solution, preparation method and application methods thereof
A technology of immune cells and storage solution, which is applied in the field of immune cell storage, can solve the problems of low immune cell function and low immune cell activity, and achieve the effect of convenient clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
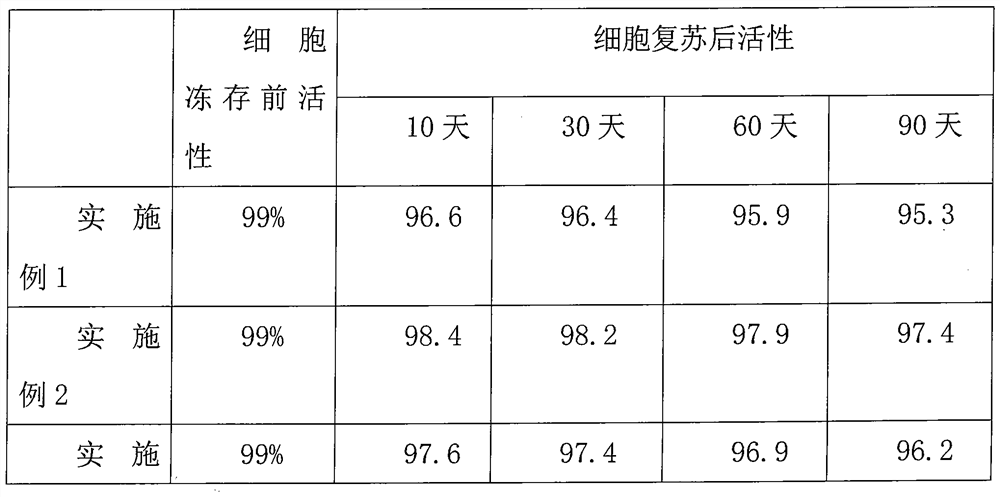
Examples
Embodiment 1
[0047] The invention provides an immune cell storage solution, each 1000ml storage solution includes the following components and contents:
[0048] Sucrose 25g;
[0049] Glycerin 50ml;
[0050] Albumin 20g;
[0051] Sodium bicarbonate 5g;
[0052] Cholesterol 0.2g;
[0053] Propylene glycol 5% mg;
[0054] Vitamin E0.6g;
[0055] Vitamin C0.6mg;
[0056] EDTA at 0.01% mg;
[0057] Phosphate buffer is 65% ml;
[0058] Sodium selenite is 0.01% mg;
[0059] The balance is cell culture medium.
Embodiment 2
[0061] The invention provides an immune cell storage solution, each 1000ml storage solution includes the following components and contents:
[0062] 55g of sucrose;
[0063] Glycerin 100ml;
[0064] Albumin 25g;
[0065] Sodium bicarbonate 7g;
[0066] Cholesterol 0.3g;
[0067] Propylene glycol 6% mg;
[0068] Vitamin E1g;
[0069] Vitamin C0.7mg;
[0070] 0.2% mg of EDTA;
[0071] Phosphate buffer is 75% ml;
[0072] Sodium selenite is 0.2% mg;
[0073] The balance is cell culture medium.
Embodiment 3
[0075] The invention provides an immune cell storage solution, each 1000ml storage solution includes the following components and contents:
[0076] 75g of sucrose;
[0077] Glycerin 150ml;
[0078] Albumin 30g;
[0079] Sodium bicarbonate 10g;
[0080] Cholesterol 0.4g;
[0081] Propylene glycol 8% mg;
[0082] Vitamin E1.1g;
[0083] Vitamin C0.9mg;
[0084] EDTA at 0.4% mg;
[0085] Phosphate buffer is 95% ml;
[0086] Sodium selenite is 0.5% mg;
[0087] The balance is cell culture medium.
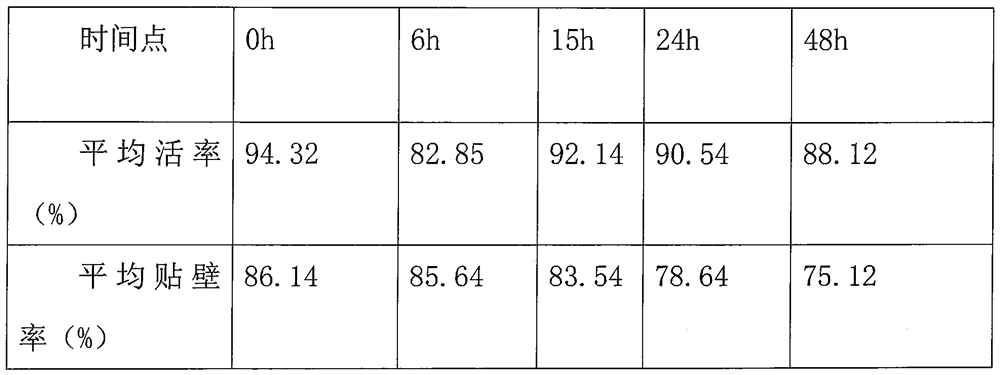
[0088] In three embodiments, the effect of each composition is as follows:
[0089] Albumin mainly regulates the dynamic balance of water in cells, maintains the osmotic pressure of cell solution, and is also a non-permeable cryoprotectant.
[0090] Propylene glycol (1,2-Propanediol, Propylene Glycol), a permeable cryoprotectant, can diffuse through the cell membrane and penetrate into the cell, balance the osmotic pressure inside and outside the cell, reduce the concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com