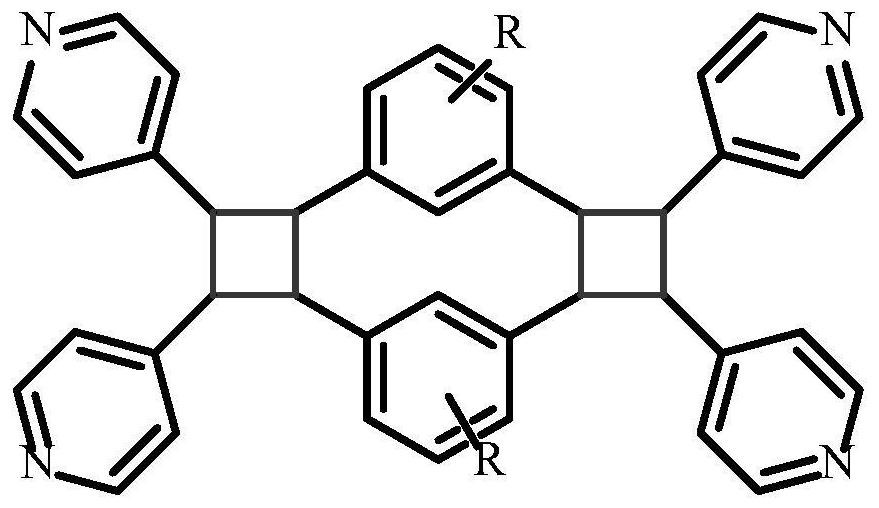
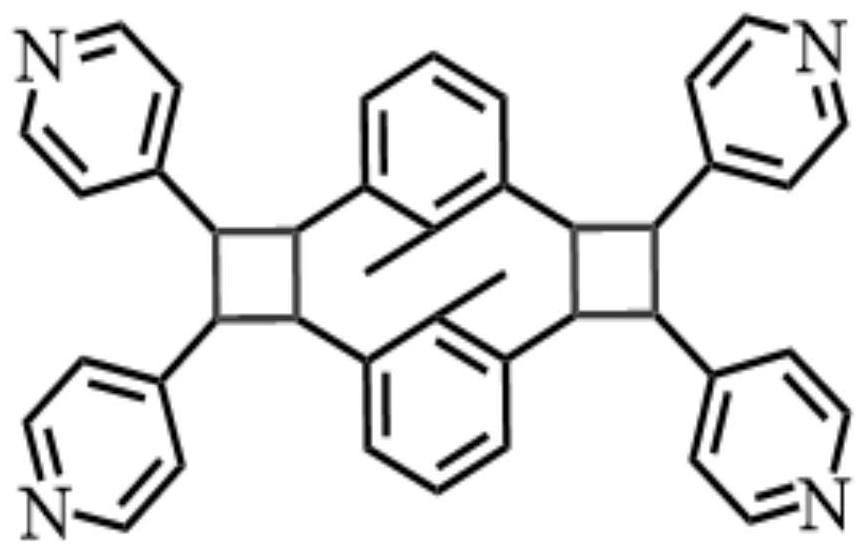
Nitrogenous bicyclobutane derivative, and preparation method and antitumor activity thereof
A bicyclobutane and derivative technology, applied in the field of synthesis of cyclobutane skeleton compounds, can solve problems such as isomerism, low efficiency, and difficult cyclization reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
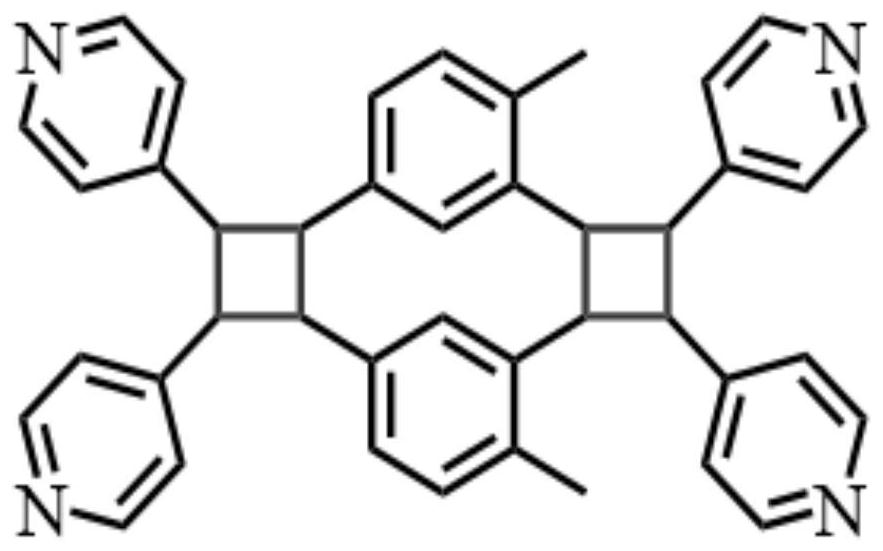
[0062]Preparation of 4-methyl-1,3-bis(4-vinylpyridine)benzene (ie: 4-CH3-1,3-bpeb)
[0063]Reaction step: (1) The principle of the HECK reaction, the halogenated hydrocarbon and the activated unsaturated hydrocarbon under palladium catalysis, the transfection was produced; 2.50 g (0.01 mol) of 2,4-dibromobenzene, 2.1g (0.02) Mol) 4-vinylpyridine, 0.2 g (0.00028 mol) palladium catalyst, 2.76 g (0.02 mol) of potassium anhydrous carbonate in 30 ml of DMF solvent, under 95 ° C oil bath for intra-nucleation replacement for 3 hours To generate a target product;
[0064](2) The target product was collected after the reaction was stopped, diluted with water (300 mL), and extracted with dichloromethane, combined with an organic layer, dried with anhydrous sodium sulfate, and the solvent was evaporated. Solid powder. The chemical structural formula of the product is obtainedFigure 8 Indicated.
[0065]Yield: Based on 2,4-di bromophenyl dosage, calculation 4-CH3The yield of -1,3-BPEB is 90%.
[0066]4-ch3...
Embodiment 2
[0068]Preparation of bisolenic ligand 2-methyl-1,3-di (4-vinylpyridine) benzene (ie: 2-ch3-1, 3-BPEB)
[0069]Reaction Step: (1) The principle of the reaction of the HECK reaction, the halogenated hydrocarbon and the activated unsaturated hydrocarbons were used under palladium catalysis, and the transfection was produced; 2.50 g (0.01 mol) of 2,6-dibromobromellin, 2.1g (0.02) Mol) 4-vinylpyridine, 0.2 g (0.00028 mol) palladium catalyst, 2.76 g (0.02 mol) of potassium anhydrous carbonate in 30 ml of DMF solvent, under 92 ° C oil bath for nucleophilic replacement reaction for 2.5 hours To generate a target product;
[0070](2) The target product was collected after the reaction was stopped, diluted with water (300 mL), and extracted with dichloromethane, combined with an organic layer, dried with anhydrous sodium sulfate, and the solvent was evaporated. Solid powder. The chemical structural formula of the product is obtainedFigure 9 Indicated.
[0071]Yield: Based on 2,6-di bromophenyl dosage,...
Embodiment 3
[0074]Preparation of double olefin ligand 4,6-methyl-1,3-di (4-vinylpyridine) benzene (ie: 4, 6-CH)3-1, 3-BPEB)
[0075]Reaction step: (1) The principle of the HECK reaction, the halogenated hydrocarbon and the activated unsaturated hydrocarbon under palladium catalysis, the transfection was produced; 2.50 g (0.01 mol) of 1,3-dimethyl-4, 6- Dibromobenzene, 2.0 g (0.02 mol) of 4-vinylpyridine, 0.2 g (0.00028 mol) palladium catalyst, 2.76 g (0.02 mol) of anhydrous potassium carbonate in 30 ml of DMF solvent, from 94 ° C oil bath The next nucleophilic replacement reaction for 2.8 hours, that is, the target product is generated;
[0076](2) The target product was collected after the reaction was stopped, diluted with water, extracted with dichloromethane, add activated carbon and heat the dichloromethane phase to remove impurities such as catalysts, dried with anhydrous sodium sulfate, vacuum evaporation solvent Solid powder. The chemical structural formula of the product is obtainedFigure 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com