Clinical NK cell cryopreservation liquid and cryopreservation method
A technology of NK cells and cryopreservation method, which is applied in the field of clinical NK cell cryopreservation solution and cryopreservation, which can solve the problems of inability to meet the needs of mass production, limited sources of human autologous serum, and increased introduction of animal-derived components. Achieve the effect of maintaining the activity and killing ability of frozen cells, improving the activity and killing ability of frozen cells, and protecting mechanical damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
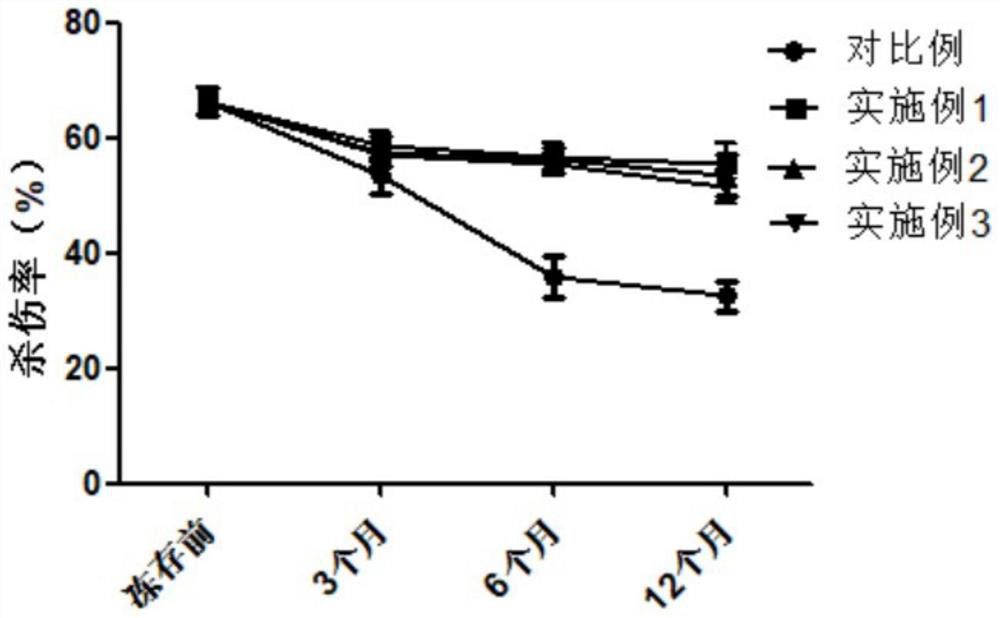
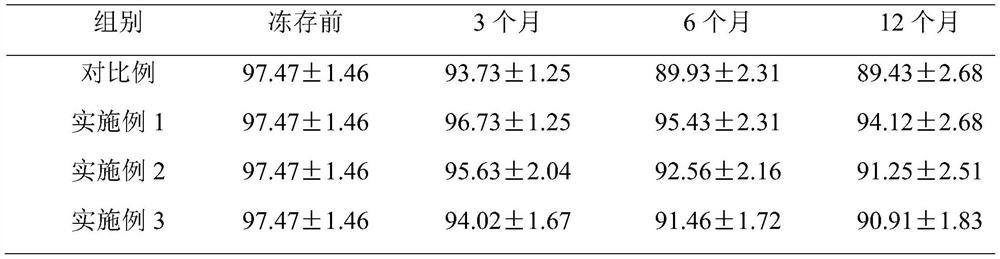
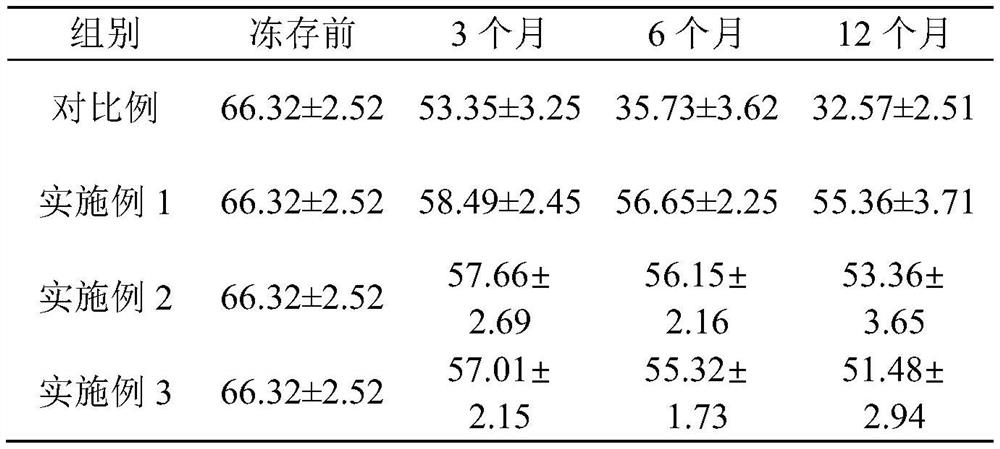
Image
Examples
Embodiment 1
[0046]Prepare NK cell cryopreservation solution for clinical use according to the following steps:
[0047]Step 1: Weigh the following components according to the volume ratio: glycerol 3%, dextran 15%, polyvinylpyrrolidone 0.1%, N-acetylcysteine 8%, glucose solution and half of water, and mix well to obtain A liquid;
[0048]Step 2: Mix DMSO and remaining water thoroughly to obtain liquid B.
[0049]Step 3: Store the solution A and solution B obtained in step 1 and step 2 at 2°C for 30 min in the dark.
[0050]Step 4: Mix the solution A and solution B obtained in step 3, add NK cells, and gently pipette until the density of the cell suspension is 8×107cells / ml; The process of NK cell processing is as follows: Centrifuge the NK cell suspension at 300g for 8 minutes, and discard the supernatant.
[0051]Step 5: Place the cell suspension in Step 4 in the dark at 4°C for 8 minutes, then cool it down, and transfer it to liquid nitrogen for storage.
[0052]Transfer to a program cooling box, place it at...
Embodiment 2
[0054]Prepare NK cell cryopreservation solution for clinical use according to the following steps:
[0055]Step 1: Weigh the following components according to the volume ratio: glycerol 3%, dextran 15%, polyvinylpyrrolidone 0.1%, N-acetylcysteine 8%, glucose solution and half of water, and mix well to obtain A liquid;
[0056]Step 2: Mix DMSO and remaining water thoroughly to obtain liquid B.
[0057]Step 3: Store the solution A and solution B obtained in step 1 and step 2 in the dark at 8°C for 30 minutes.
[0058]Step 4: Mix liquid A and B obtained in step 3, add NK cells, and gently pipette until the density of the cell suspension is 1×107cells / ml; The process of NK cell processing is as follows: Centrifuge the NK cell suspension at 300g for 8 minutes, and discard the supernatant.
[0059]Step 5: Place the cell suspension in Step 4 in the dark at 6°C for 8 minutes, then cool it down, and transfer it to liquid nitrogen for storage.
[0060]Transfer to a program cooling box, place it at -80°C, and...
Embodiment 3
[0062]Prepare NK cell cryopreservation solution for clinical use according to the following steps:
[0063]Step 1: Weigh the following components according to the volume ratio: glycerol 3%, dextran 15%, polyvinylpyrrolidone 0.1%, N-acetylcysteine 8%, glucose solution and half of water, and mix well to obtain A liquid;
[0064]Step 2: Mix DMSO and remaining water thoroughly to obtain liquid B.
[0065]Step 3: Store the solution A and solution B obtained in step 1 and step 2 at 6°C for 30 minutes in the dark.
[0066]Step 4: Mix the solution A and solution B obtained in step 3, add NK cells, and gently pipette until the density of the cell suspension is 8×107cells / ml; The process of NK cell processing is as follows: Centrifuge the NK cell suspension at 300g for 8 minutes, and discard the supernatant.
[0067]Step 5: Place the cell suspension in Step 4 in the dark at 4°C for 8 minutes, then cool it down, and transfer it to liquid nitrogen for storage.
[0068]Transfer to a program cooling box, place i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com