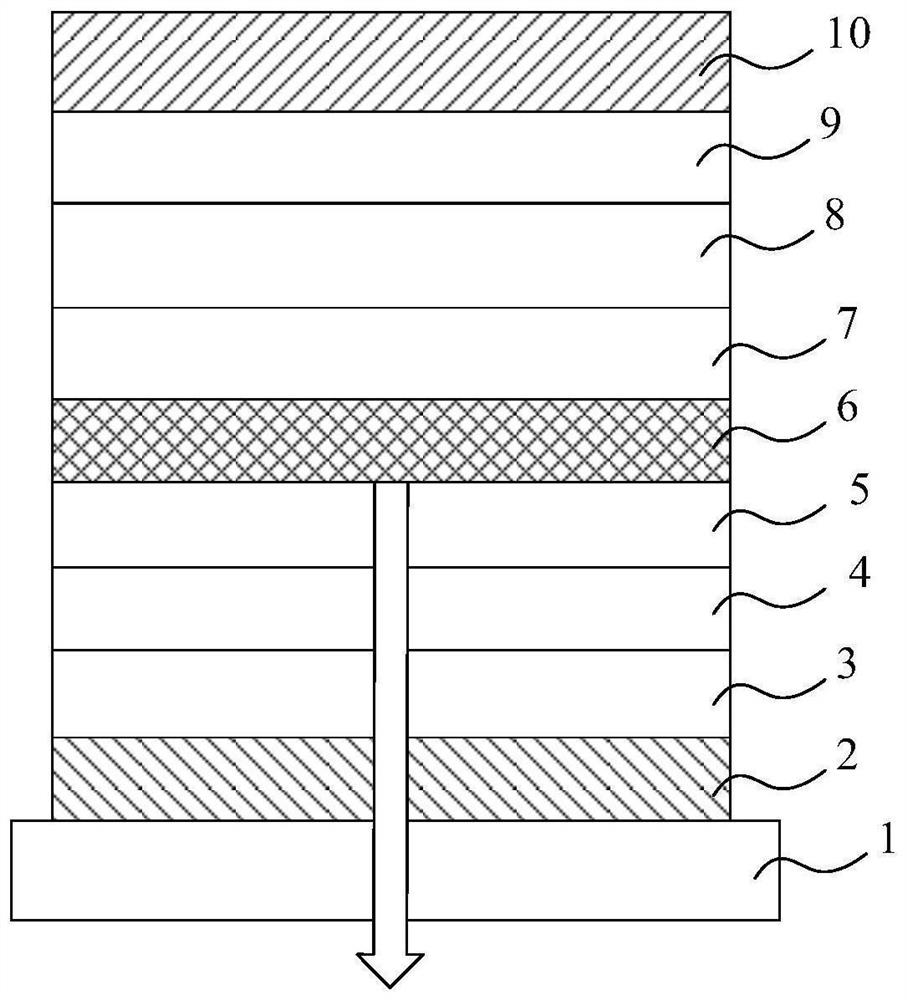
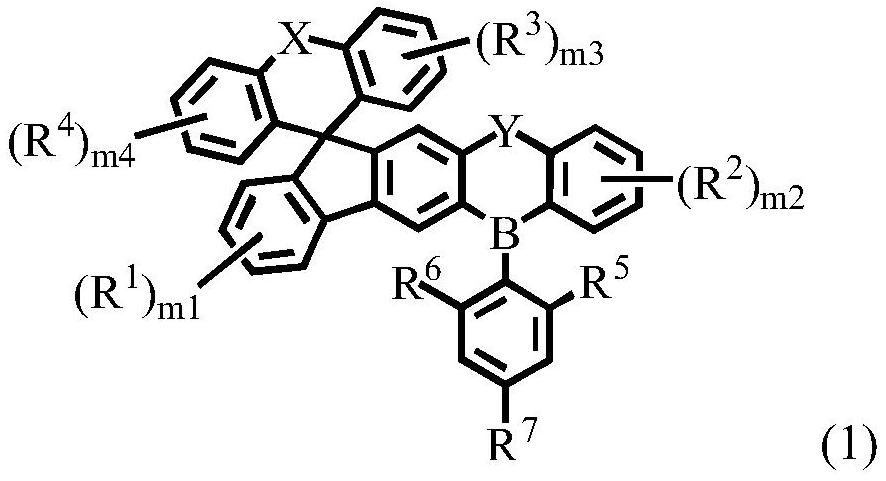
Compound, display panel and display device
A compound, independent technology, applied in the fields of compounds containing Group 3/13 elements of the periodic table, organic chemistry, chemical instruments and methods, etc., can solve the problems of efficiency roll-off of phosphorescent materials, disadvantageous mass production, etc., and achieve high The effect of luminous efficiency and efficient exciton recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Embodiment 1: the preparation of compound 1
[0175]
[0176] Under a nitrogen atmosphere, add about 100mL of anhydrous toluene to a 250mL reaction flask, then add reactant A1 (4mmol), reactant a-1 (4mmol), sodium tert-butoxide (10mmol), catalyst Pd 2 (dba) 3 (0.2mmol) and the ligand S-Phos (0.6mmol), the temperature was raised to 110°C, and the reaction was carried out overnight. After the reaction is complete, cool to room temperature, add dichloromethane / H 2 O was extracted, and the collected organic phase was washed with anhydrous Na 2 SO 4 After drying, the filtrate was collected by suction filtration, the solvent was spun off and purified by column chromatography to obtain intermediate B1 (yield 81%). MALDI-TOF: m / z: Calculated: C31H18BrIO: 611.96, Found: 612.17.
[0177]
[0178] Under a nitrogen atmosphere, add the reaction solvent 1,2-dichlorobenzene to the reaction flask, and then add the reaction intermediate B1 (2mmol), reactant b-1 (2mmol), potas...
Embodiment 2
[0184] Embodiment 2: the preparation of compound 2
[0185]
[0186] The difference between the preparation method of intermediate C2 and the step (2) of Example 1 is that compound b-1 is replaced with an equimolar amount of compound b-2, and other raw materials, reaction steps and reaction conditions are the same as in Example 1, and finally intermediate Body C2 (yield 76%). MALDI-TOF: m / z: Calculated: C55H33BrN2O: 816.18, Found: 816.39.
[0187]
[0188] The difference between the preparation method of this compound 2 and the step (3) of Example 1 is that the intermediate C1 is replaced with an equimolar amount of compound C2, and other raw materials, reaction steps and reaction conditions are the same as in Example 1, and finally compound 2 (yield rate of 67%).
[0189] MALDI-TOF: m / z: Calculated: C64H43BN2O: 866.35, Found: 866.54.
[0190] Compound elemental analysis results: calculated value: C64H43BN2O (%): C 88.68, H 5.00, N 3.23; test value: C 88.69, H 4.99, N...
Embodiment 3
[0191] Embodiment 3: the preparation of compound 3
[0192]
[0193] The difference between the preparation method of this intermediate C3 and the step (2) of Example 1 is that the compound b-1 is replaced with an equimolar amount of compound b-3, and other raw materials, reaction steps and reaction conditions are the same as in Example 1, and finally obtained Intermediate C3 (75% yield). MALDI-TOF: m / z: Calculated: C46H32BrNO: 693.17, Found: 693.38.
[0194]
[0195] The difference between the preparation method of this compound 3 and the step (3) of Example 1 is that the intermediate C1 is replaced with an equimolar amount of compound C3, and other raw materials, reaction steps and reaction conditions are the same as in Example 1, and finally compound 3 (yield rate of 66%).
[0196] MALDI-TOF: m / z: Calculated: C55H42BNO: 743.34, Found: 743.56.
[0197] Compound elemental analysis results: calculated value: C55H42BNO (%): C 88.82, H 5.69, N 1.88; test value: C 88.84,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com