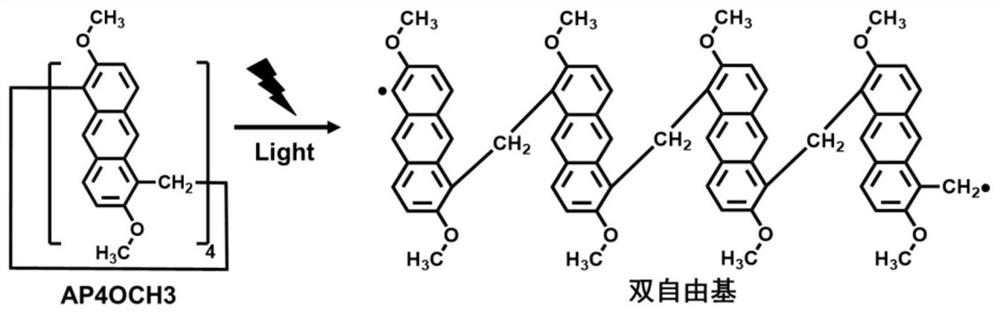
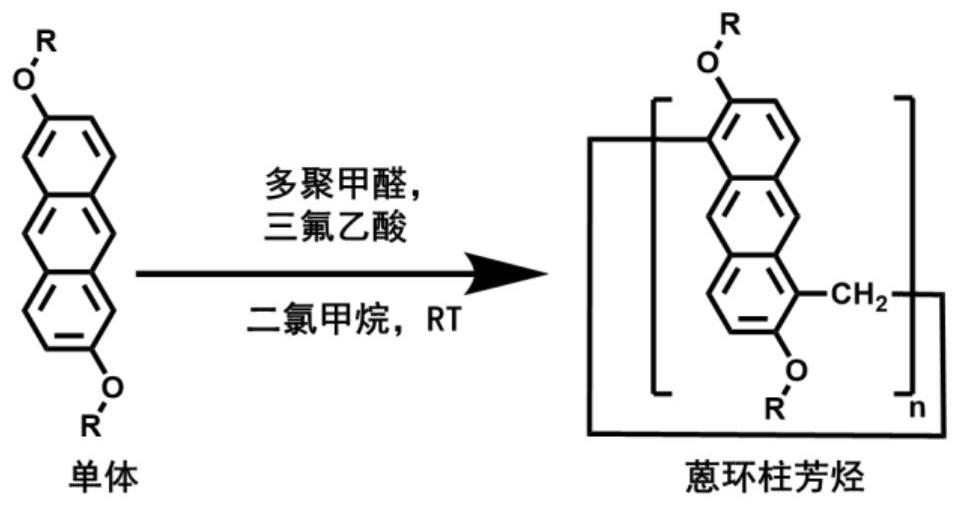
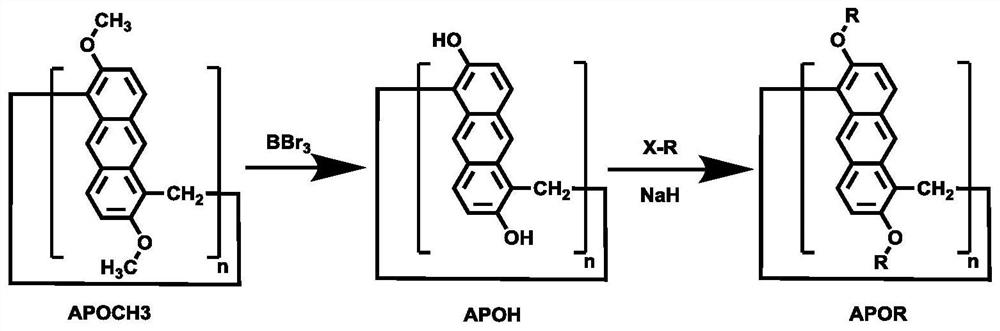
Anthracene ring pillar aromatic hydrocarbon initiator and preparation method thereof
An anthracycline pillar aromatic hydrocarbon and pillar aromatic hydrocarbon technology are applied in the application and preparation field of anthracene pillar aromatic hydrocarbon as a photoinitiator, and can solve the problems of strong odor, high toxicity, leaching of harmful substances and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Such as Figure 4 , Take 300 mg of dimethoxyanthracene (AOCH3), dissolve it in 100 mL of dry dichloromethane, add 117 mg of paraformaldehyde, then add 47 μL of trifluoroacetic acid, and stir at room temperature for 9 h under the protection of argon. The reaction was quenched by adding 50 mL of water, and the organic layer was spin-dried, and separated by silica gel chromatography to obtain anthracycline columnarene (AP4OCH3). The eluent is petroleum ether:ethyl acetate=1.5:1.
[0027] preferred, such as Figure 5 , we choose anthracycline columnarene (AP4OCH3) with methoxy groups at both ends as the initiator, and PEGDA with two double bonds as the monomer. Weigh 10mg AP4OCH3, dissolve it in 1g PEGDA monomer, and configure an initiator-monomer mixed system with a mass fraction of 1%. Then under the irradiation of 365nm, 385nm, 395nm and 405nm LED lamps, the photopolymerization kinetics curve was measured by real-time infrared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com