Preparation method of battery grade nickel salt
A battery-grade, nickel-salt technology, applied in the field of battery-grade nickel salt preparation, can solve the problems of the danger of explosion of hydrogen and oxygen, the lower utilization rate of the tank body, and the increase in the cost of hydrogen peroxide, so as to reduce the introduction of impurities and improve the Safety, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
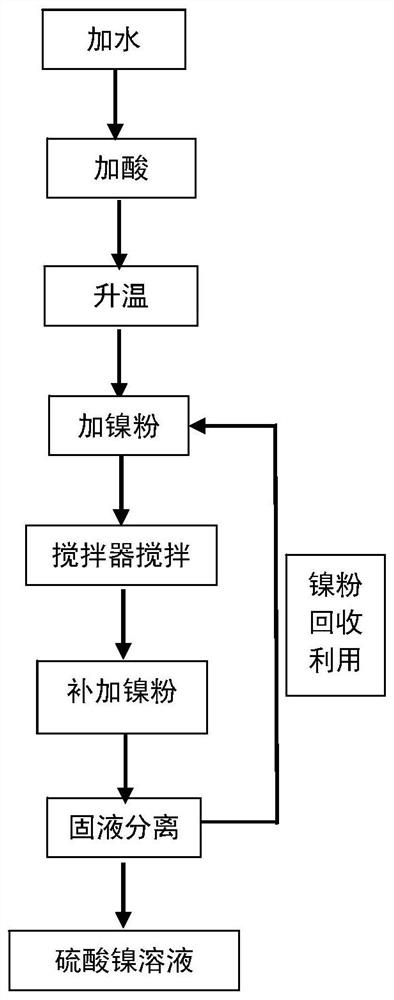
[0038] The present invention proposes a method for preparing a battery-grade nickel salt that can promote the reaction without adding hydrogen peroxide, comprising the following steps:
[0039] Add water and mineral acid into the reaction vessel to form an acid solution;
[0040] Heat the acid solution to 90-110°C, add nickel powder to react, add nickel powder during the reaction to maintain the nickel powder concentration and liquid-solid ratio of the reaction system (concentration and liquid-solid ratio can be considered as one meaning), reaction 14 -20h, when the pH value of the solution is 3-5, the reaction ends and a nickel salt solution is obtained;
[0041] Among them, the liquid-solid ratio of acid solution and nickel powder is 4-6:1ml / g, and the amount of nickel powder added is 1.8-2.2 times of the theoretical consumption of nickel powder in the amount of inorganic acid; the reaction vessel does not contain baffles.
[0042] Battery-grade nickel salt is an industry t...
Embodiment 1
[0078] According to the process flow, first measure 4450mL of water into the reaction vessel, add 550mL of 98% concentrated sulfuric acid to make a sulfuric acid solution, then heat up to 90°C, and keep the reaction temperature ≥ 90°C, add 1200g of nickel powder, and start the stirrer to stir , the stirring speed is 1000r / min, so that there is no nickel powder deposition at the bottom of the container, measure the acidity after three hours of reaction, calculate that the reaction has consumed 300g of nickel powder, then add 300g of nickel powder to it to maintain the concentration of nickel powder and the liquid-solid ratio. Measure the acidity after three hours, and calculate that 200g of nickel powder is consumed, then add 200g of nickel powder to it, maintain the concentration of nickel powder and the liquid-solid ratio, increase the reaction rate, and the total reaction time is 15-16h. The pH of the solution reaches about 4, and the reaction is stopped. After filtering, the...
Embodiment 2
[0081] According to the process flow, first measure 4550mL of water into the reaction vessel, add 450mL of 98% concentrated sulfuric acid to make a sulfuric acid solution, then heat up to 110°C and keep it, add 1000g of nickel powder, turn on the stirrer, and the stirring speed is 650r / min , make the bottom of the container without nickel powder deposition, measure the acidity after reacting for 3 hours, calculate the reaction nickel powder 250g, add 250g nickel powder to it, measure the acidity after continuing to react for 3 hours, calculate and consume about 120g of nickel powder, then add to Among them, 120g of nickel powder is added to maintain the concentration of nickel powder and the liquid-solid ratio, and the reaction rate is increased. The total reaction time is 16-17h, and the pH of the solution reaches about 4.5. Stop the reaction and filter, and the nickel powder can be returned to the reaction tank for continued use to obtain the concentration of nickel sulfate so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com