Chondroitin sulfate ABC lyase mutant with high thermal stability and application thereof
A chondroitin sulfate, mutant technology, applied in the field of bioengineering, can solve the problems of low stability, mixed chondroitin sulfate impurities, limited application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0059] The primers used in Table 2 Example 2
[0060]
[0061]
[0062] Primers used in table 3 embodiment 3
[0063]
[0064] Primers used in table 4 embodiment 4
[0065]
[0066]
Embodiment 1
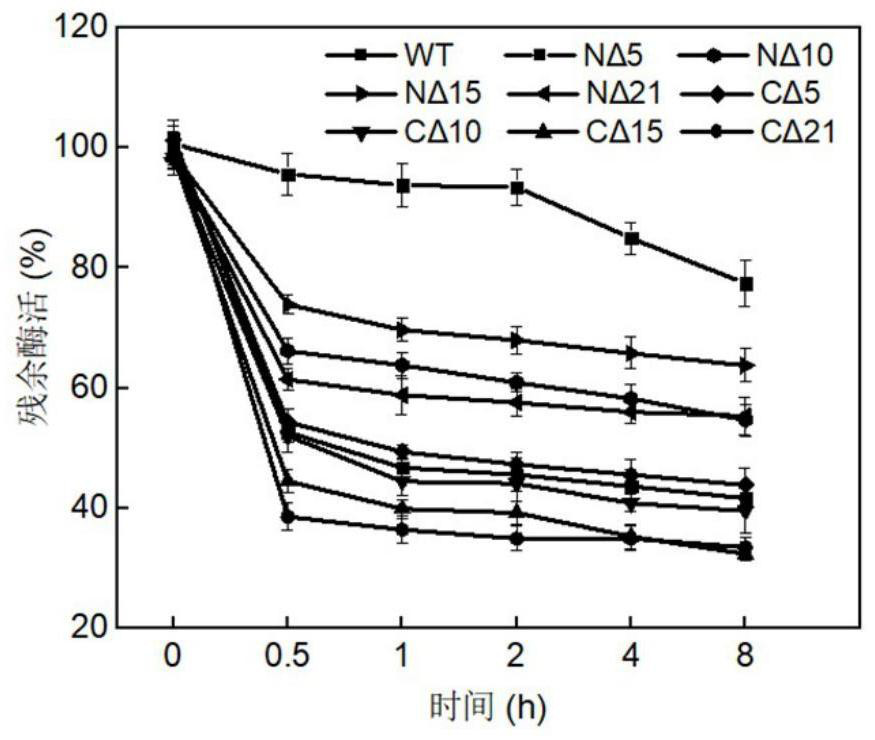
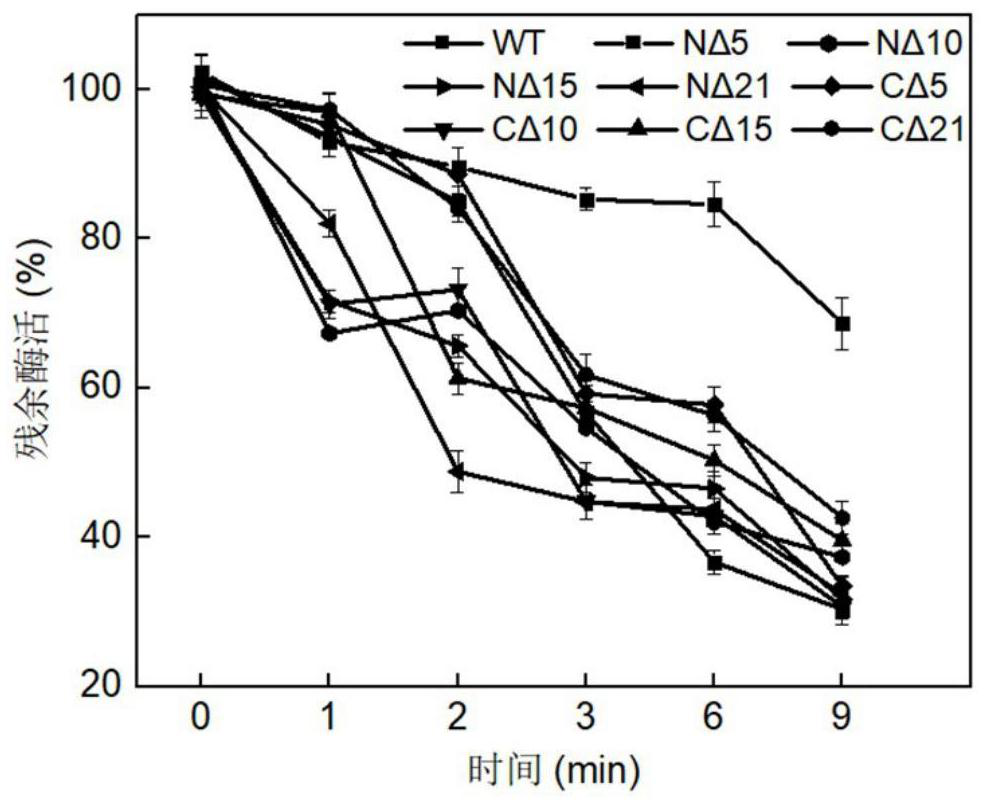
[0067] Embodiment 1: the preparation method of chondroitin sulfate ABC lyase truncation mutant
[0068] Synthesize chondroitin sulfate ABC lyase derived from Proteus vulgaris, the original amino acid sequence of the chondroitin sulfate ABC lyase is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.4. PCR was performed using the primers shown in Table 1. The truncated gene was connected to the XhoI and NdeI restriction sites of the pET-28a plasmid, introduced into Escherichia coliBL21 (DE3), cultured at 37°C until a single clone was grown, and the single clone was picked for sequencing to verify that it was correct The positive transformant is the recombinant Escherichia coli strain expressing the chondroitin sulfate ABC lyase mutant.
[0069] The obtained recombinant strain was inoculated in LB medium, cultured overnight at 37°C, transferred to a 250mL Erlenmeyer flask filled with LB medium, cultured at 30°C for 1 hour, induced by adding IPTG with a fina...
Embodiment 3
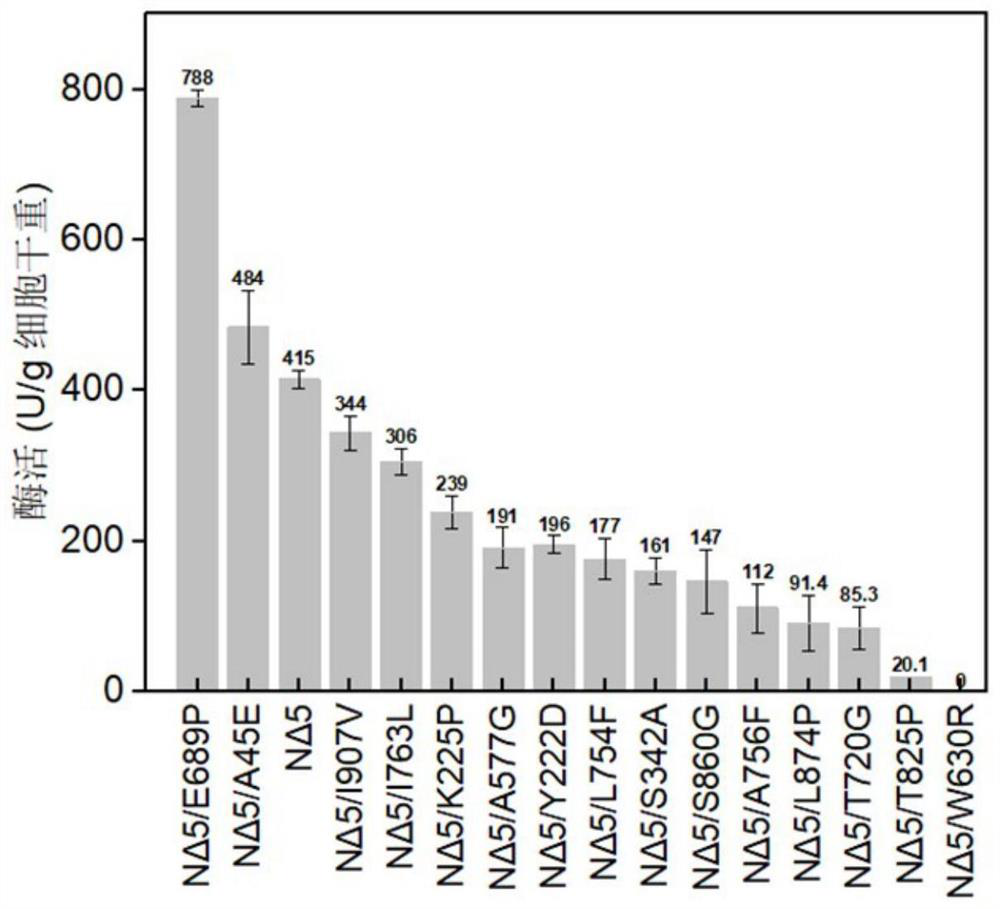
[0084] Embodiment 3: Preparation of chondroitin sulfate ABC lyase saturation mutant
[0085] Saturation mutation of NΔ5: using the recombinant plasmid contained as a template, through saturation mutation primers (see Table 4 for the sequence of saturation mutation primers), amplify the entire plasmid of the target sequence to obtain a circular plasmid with a cross gap, and digest it with Dpn I fast cutting enzyme The template plasmid and the digestion solution were directly transformed into E.coli BL21 (DE3) competent cells, and the single clone was extracted and verified by plasmid sequencing.
[0086] The recombinant Escherichia coli strains with correct sequencing were inoculated in LB medium, cultured overnight at 37°C, then transferred to a 250mL Erlenmeyer flask filled with LB medium, cultured at 30°C for 1 hour, and induced by adding IPTG with a final concentration of 0.5mM, Placed at 27°C for 20h. After the cultivation is completed, centrifuge the fermentation broth a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com