Transdermal Penetration Enhancement Composition and Its Application in Timolol Preparation
A technology of timolol and composition, applied in the application field of timolol preparations, can solve the problems such as the absence of hemangioma disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] 1. Preparation of Ex vivo Skin
[0040] Get the male piglet (age: 1 month), remove the hair from the abdomen with an electric razor, and apply the moisturizing agent glycerin solution to the skin at the depilated place. Execute before the test, take the hairless skin to remove the subcutaneous fat, rinse it with distilled water repeatedly until it is clean, and use filter paper to blot the remaining distilled water on the skin surface to obtain the final product.
[0041] 2. Ex vivo transdermal device and method
[0042] The improved Franz vertical diffusion cell was used for transdermal experiment. The stirrer was placed in the receiving tank, and the pig skin (pig abdomen skin) was placed between the supply chamber and the receiving tank. The dermis faced the receiving liquid and fixed. Use the receiving tank as the receiving solution, and then apply about 0.5g of the test product on the pigskin, the effective penetration area is 3.14cm 2 . Place the diffusion cell...
Embodiment 1
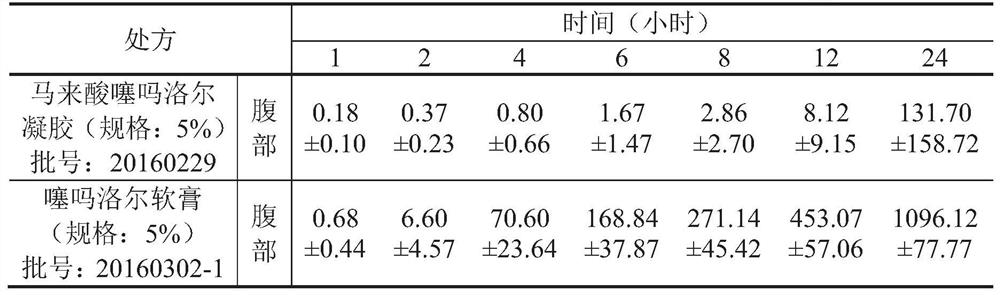
[0045] The transdermal effect contrast of embodiment 1 timolol maleate and timolol
[0046] Timolol maleate gel preparation process:
[0047] Weigh 2% of hypromellose and 15% of glycerin and mix well and add appropriate amount of water to form the gel matrix, and another 5% of timolol maleate is dissolved completely with appropriate amount of water (heated to 50°C and stirred) To obtain timolol maleate solution, add the dissolved timolol maleate solution to the gel matrix to obtain mixture A, weigh 2% laurocaprolactone and 5% propylene glycol and mix well and then add the mixture In A, stir evenly to obtain a timolol maleate gel sample.
[0048] Timolol ointment preparation process:
[0049] Weigh 5% white beeswax and 88% white petrolatum in a suitable container, heat in a water bath at 80°C to melt, and stir to mix evenly to obtain mixture A; after lowering the temperature of mixture A slightly, weigh 5% timolol Er and 2% laurocaprazine were added to the mixture A, stirred...
Embodiment 2
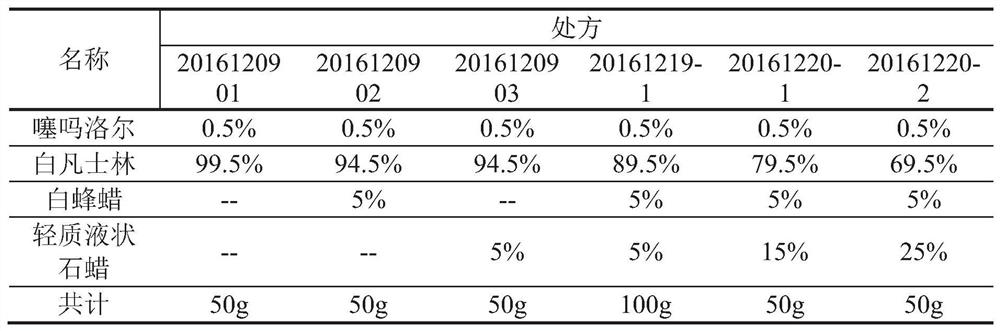
[0053] The transdermal effect contrast of embodiment 2 various timolol ointment prescriptions
[0054] As shown in Table 2, weigh light liquid paraffin, white vaseline and white beeswax in the prescribed amount in a suitable container, and put them in a water bath at 80°C until they are completely melted to obtain mixture A. Cool the mixture A to 65°C, keep it warm, weigh the prescribed amount of timolol and add it to the mixture A, stir for 6 minutes to dissolve completely, continue to stir and cool to room temperature to get final product.
[0055] Table 2 Various timolol ointment prescriptions
[0056]
[0057] The above-mentioned 6 prescription samples were subjected to transdermal test, and the influence of different prescriptions on drug transdermal behavior was investigated through piglet abdominal skin in vitro transdermal test. 4 mL of receiving solution was taken out at 1, 2, 4, 6 and 8 hours respectively, and at the same time Supplement with the same volume of b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com