Methods for preparing 1,4-dithiene and thiophene compounds from elemental sulfur and active internal alkyne through temperature regulation and control, and conversion reaction of 1,4-dithiene compounds into thiophene compounds
A compound, dithiene technology, applied in organic chemistry and other directions, can solve the problems of non-green environmental protection, single structure, harsh conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
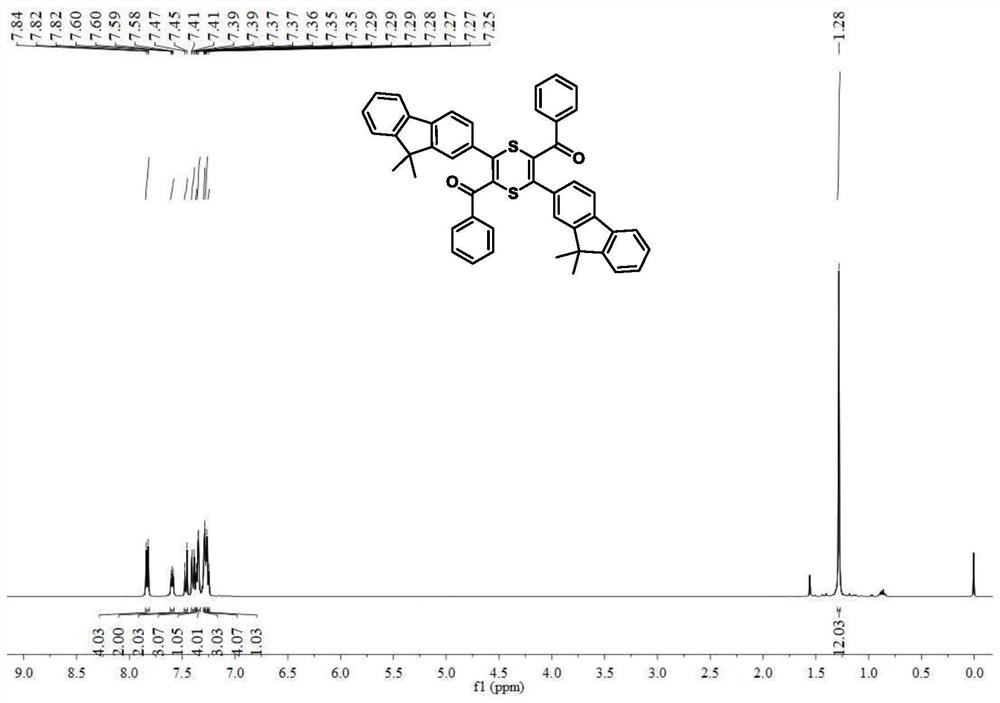
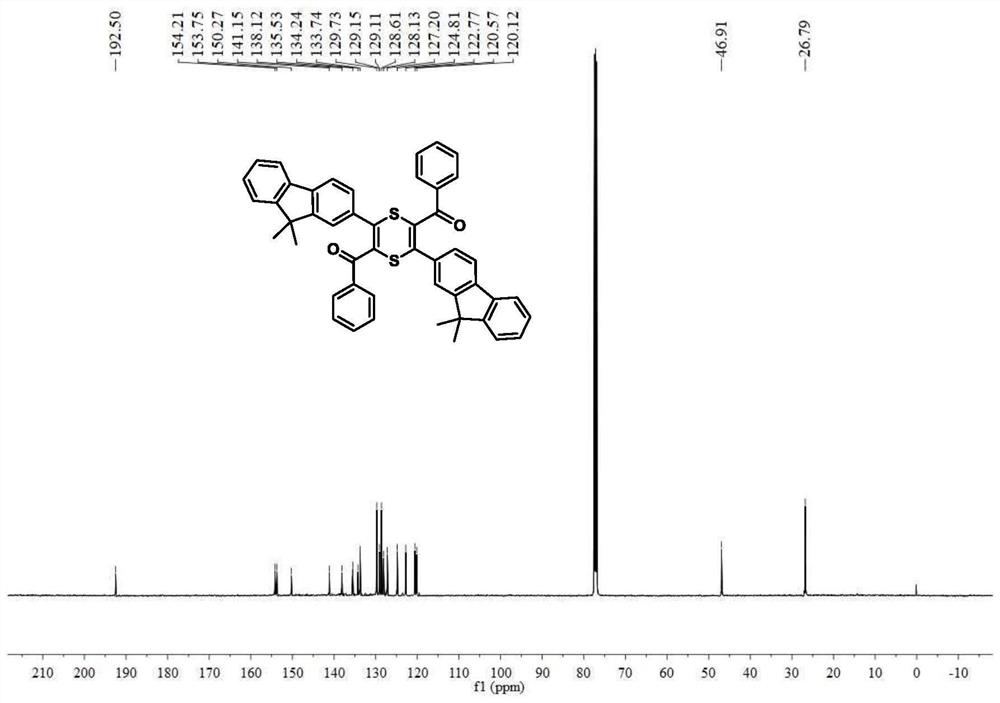
[0078] The 2,5-dibenzoyl-3,6-difluorenyl-1,4-dithiene 3a and 2,6-dibenzoyl-3,5-difluorenyl-1,4-di Thiene 3b compound is prepared by direct reaction of acetylenone and elemental sulfur, and the reaction equation is as formula (1):
[0079]
[0080] Formula (1)
[0081] 1 is sublimed sulfur, which can be purchased from the market, in this example, it was purchased from Guangzhou brand chemical reagents. 2 is acetylene, and its synthesis method is as described in the literature (Macromolecules 2015,48,1941-1951);
[0082] The preparation steps of the 1,4-dithienes are as follows:
[0083] Add 0.32g (1.0mmol) of acetylene compound, 0.10g (3.0mmol) of elemental sulfur, and 0.06g (1.0mmol) of potassium hydroxide to a 10ml polymerization tube, and inject 2.0mL of dimethylmethylene Sulfone, react at room temperature for 2 hours, point the plate to detect the progress of the reaction, after the reaction is completed, add saturated NaCl solution to the reaction solution, extract w...
Embodiment 2
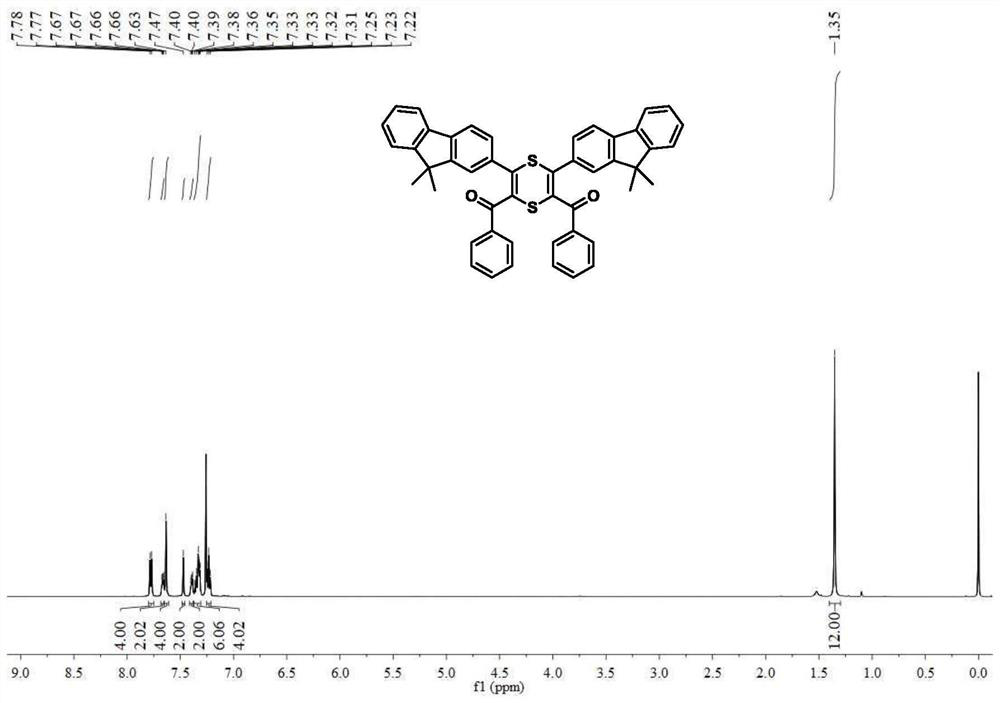
[0096] The thiophene compound is prepared by the direct reaction of acetylenone and elemental sulfur, and the reaction equation is as formula (2):
[0097]
[0098] Formula (2)
[0099] 1 is sublimed sulfur, which can be purchased from the market, in this example, it was purchased from Guangzhou brand chemical reagents. 2 is acetylene, and its synthesis method is as described in the literature (Macromolecules 2015,48,1941-1951);
[0100] The preparation steps of the 2,4-dibenzoyl-3,5-difluorenylthiophene 4a and 2,5-dibenzoyl-3,4-difluorenylthiophene 4b compounds are as follows:
[0101] Add 0.32g (1.0mmol) of acetylene compound, 0.10g (3.0mmol) of elemental sulfur, and 0.06g (1.0mmol) of potassium hydroxide to a 10ml polymerization tube, and inject 2.0mL of dimethylmethylene Sulfone, react at 80 degrees for 2 hours, point the plate to detect the progress of the reaction, after the reaction is completed, add saturated NaCl solution to the reaction solution, extract with wa...
Embodiment 3
[0114] The 1,4-dithienes can be converted to thiophenes by heating, and the reaction equation is as formula (3):
[0115]
[0116] Formula (3):
[0117] Experimental procedure for converting the 2,5-dibenzoyl-3,6-difluorenyl-1,4-dithiene 3a into 2,4-dibenzoyl-3,5-difluorenylthiophene 4a as follows:
[0118] Add 0.35g (0.5mmol) 1,4-dithiene 3a to a 10ml polymerization tube, inject 2.0mL dimethyl sulfoxide with a syringe, react at 80°C for 4 hours, spot the plate to detect the progress of the reaction, wait until After the reaction, extract with water and dichloromethane, remove the organic phase of the lower layer, dry the organic phase over anhydrous magnesium sulfate to remove water, filter to get the filtrate, distill off the dichloromethane under reduced pressure, obtain the crude product, and purify it by column chromatography , the eluent used was a mixed solvent of petroleum ether:dichloromethane with a volume ratio of 2:1, and the yield of thiophene 4a was 75%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com