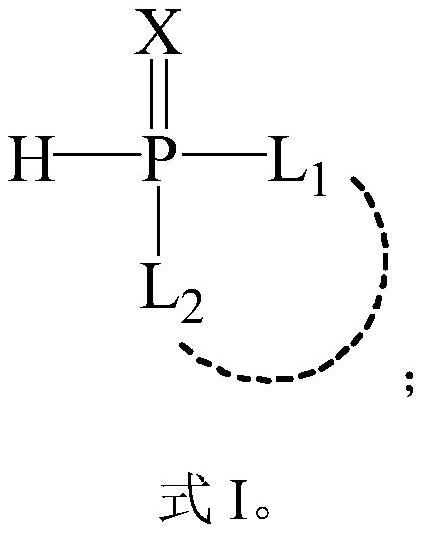
Reactive flame retardant with carboxylic acid or anhydride groups as well as preparation method and application of reactive flame retardant
A reactive flame retardant and acid anhydride technology, applied in the field of flame retardants, can solve the problems of expensive raw materials, narrow application range, and inability to be widely used, and achieve the effect of simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
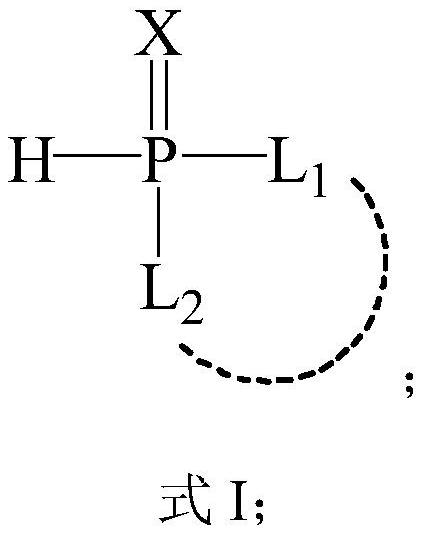
[0029] A reactive flame retardant with a carboxyl group, the structure is as follows:
[0030]
[0031] The preparation method is as follows:
[0032] Add 1mol of DOPO, 1mol of glutaconedic acid, and 50mL of glacial acetic acid into a three-necked flask equipped with magnetic stirring and a thermometer. Add the glacial acetic acid solution containing 1mmol of Pb catalyst dropwise at 50°C under stirring. In the reaction system, the reaction was carried out for 12 hours, and the product was separated to obtain the reactive flame retardant with the above structure.
[0033] 1 H NMR (CDCl 3 ,400MHz,TMS):δ=6.94-7.64(m,8H,ph-H),2.60-2.64(d,4H,COOH-C H 2 -CH-C H 2 -COOH),1.90-1.94(m,1H,-CH-).
preparation example 2
[0035] A reactive flame retardant with an anhydride group, the structure is as follows:
[0036]
[0037] The preparation method is as follows:
[0038] Under a nitrogen atmosphere, put 50mL of glacial acetic acid, 0.5mol dimethyl phosphite and 0.5mol maleic anhydride into a three-port 200mL glass reactor with a stirring device and stir. At a stirring temperature of 60°C, 0.5mmol of Pb In 20mL of glacial acetic acid, then added dropwise to the reaction system, reacted for 12h, and then separated the product to obtain the phosphorus-containing flame retardant with the above structure.
[0039] 1 H NMR (CDCl 3 ,400MHz,TMS):δ=4.12-4.16(t,1H,-CH-),3.73-3.76(d,6H,-P-O-CH 3 ),3.38-3.42(d,2H,-CH 2 -).
preparation example 3
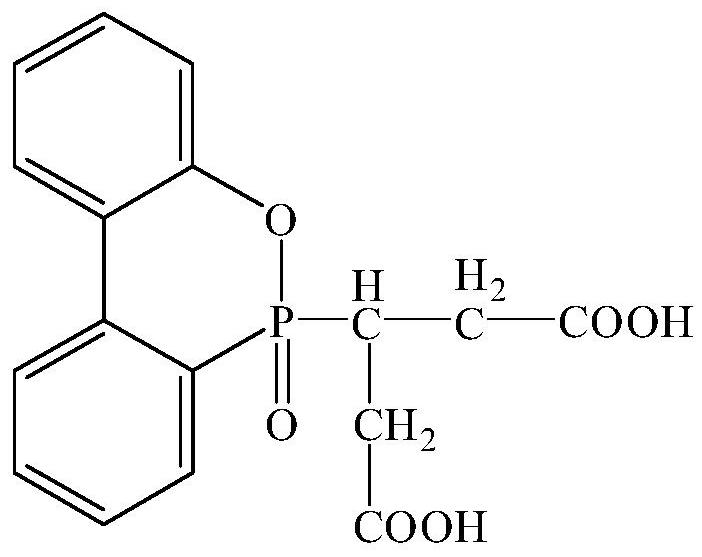
[0041] A reactive flame retardant with a carboxyl group, the structure is as follows:
[0042]
[0043] The preparation method is as follows:
[0044] Add 1 mol of dimethyl phosphite, 1 mol of glutaconedic acid, and 50 mL of glacial acetic acid into a three-necked flask equipped with magnetic stirring and a thermometer. At a stirring speed of 50 ° C, the glacial acetic acid solution containing 1 mmol of Pb catalyst is Add it dropwise into the reaction system, react for 12 hours, and separate the product to obtain the reactive flame retardant with the above structure.
[0045] 1 H NMR (400MHz, DMSO-d6): δ=10.62-10.64(s,2H,-COOH),3.73-3.76(d,6H,-P-O-CH 3 ),2.32-2.50(m,5H,COOH-C H 2 -C H -C H 2 -COOH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com