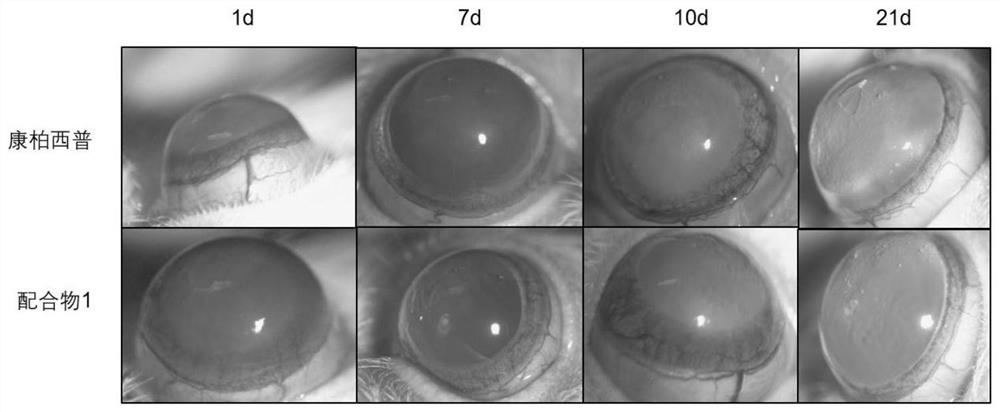
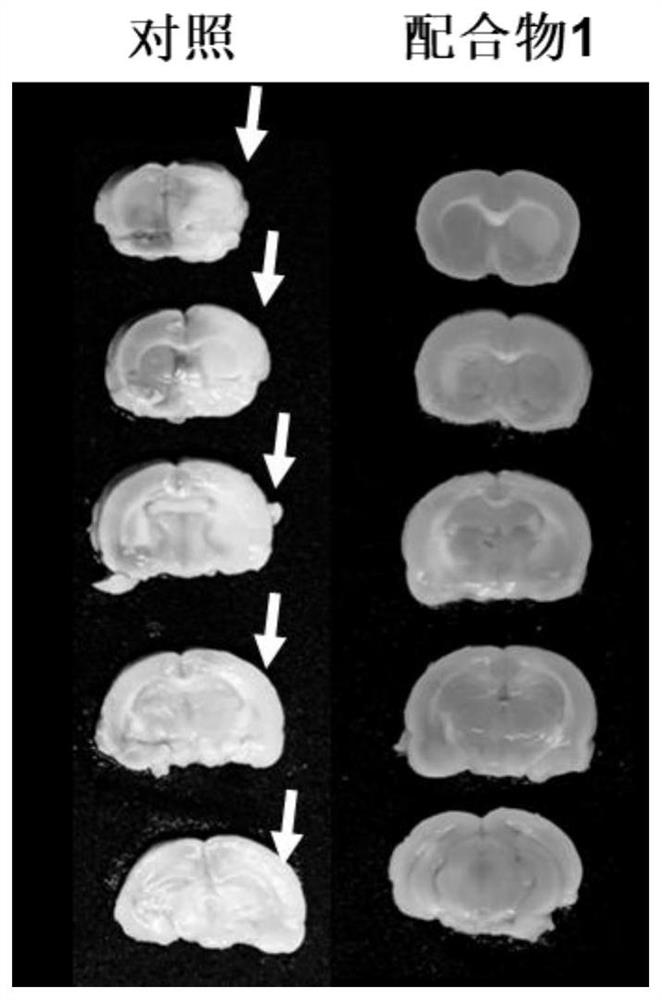
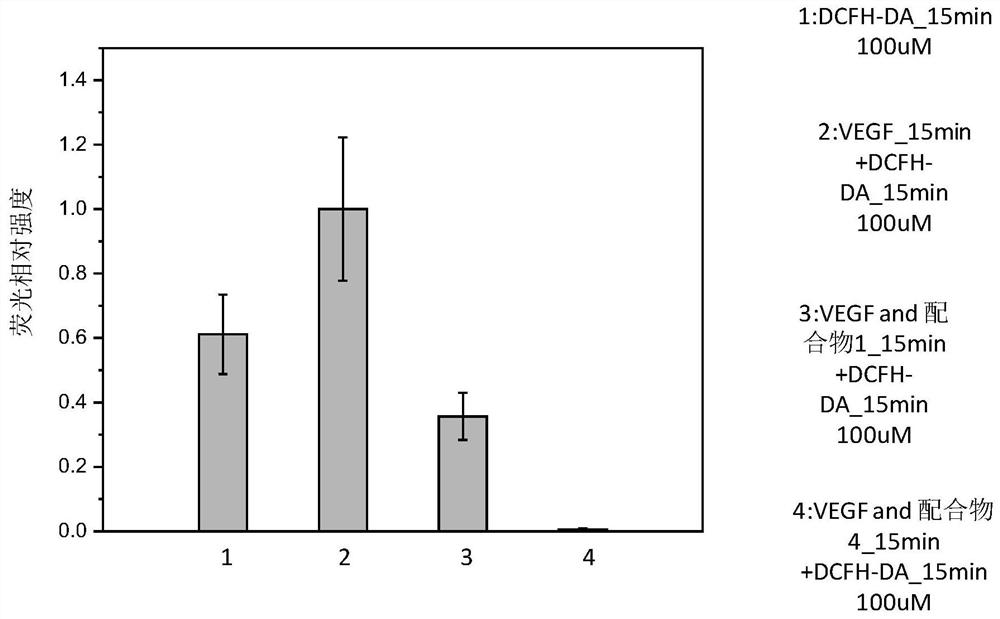
Trinuclear Schiff base metal complex and its preparation method and use
A tri-nuclear Schiff and Schiff base technology, applied to iron group organic compounds without C-metal bonds, 1/11 group organic compounds without C-metal bonds, and 2/12 group organic compounds without C-metal bonds Compounds and other directions can solve the problems of limited application and no biological activity research, and achieve the effects of inhibiting angiogenesis, excellent anti-reactive oxygen species performance, and good redox activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The present invention also provides a preparation method of the above-mentioned trinuclear Schiff base metal organic complex, the preparation method comprising the following steps:
[0066] 1) under an inert atmosphere, under catalytic conditions, anisole and ethyl chloroformate react in a first organic solvent to obtain a first intermediate product;
[0067] 2) reacting the first intermediate product with p-toluenesulfonic acid in a second organic solvent to obtain a second intermediate product;
[0068] 3) under catalytic conditions, reacting the second intermediate product with N,N-dimethylformamide in a third organic solvent to obtain a third intermediate product;
[0069] 4) In the fourth organic solvent, remove the methyl group on the methoxy group of the third intermediate product to obtain the fourth intermediate product;
[0070] 5) Condensing the fourth intermediate product with the diamine in the fifth organic solvent, and then performing ligand replacement ...
Embodiment 1
[0130] Synthesis of complex 1:
[0131]
[0132] Mix anisole and ethyl chloroformate in anhydrous ether, and react under nitrogen atmosphere at 0-10°C to obtain the first intermediate product; the first intermediate product is mixed with p-toluene at room temperature (0°C-30°C) Sulfonic acid is reacted in acetonitrile and tetrahydrofuran mixed solvent to obtain the second intermediate product; the second intermediate product is reacted with N,N-dimethylformamide in anhydrous ether to obtain the third intermediate product; the third intermediate product and three Boron bromide was reacted in dichloromethane to obtain the fourth intermediate product; the fourth intermediate product was reacted with o-phenylenediamine, then reacted with manganese acetate, and the ligand was exchanged with LiCl solution to obtain complex 1. ESI HRMSm / z=1197.03356 calcd for [C 62 h 38 Cl 2 mn 3 N 6 o 6 ] + =1197.04. Elemental analysis C, 60.59; H, 3.35; N, 6.76.
Embodiment 2
[0134] Synthesis of complex 2:
[0135]
[0136] Mix anisole and ethyl chloroformate in anhydrous ether, and react at room temperature under nitrogen atmosphere to obtain the first intermediate product; the first intermediate product is reacted with p-toluenesulfonic acid in acetonitrile and tetrahydrofuran mixed solvent at room temperature , to obtain the second intermediate product; the second intermediate product reacts with N,N-dimethylformamide in anhydrous ether to obtain the third intermediate product; the third intermediate product reacts with boron tribromide in dichloromethane to obtain The fourth intermediate product; the fourth intermediate product is reacted with 1,2-cyclohexanediamine, then reacted with manganese acetate, and the ligand is exchanged with LiCl solution to obtain complex 2. ESI HRMS m / z=1215.17518 calcd for [C 62 h 56 Cl 2 mn 3 N 6 o 6 ] + =1215.18. Elemental analysis C, 59.39; H, 4.69; N, 6.66.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com