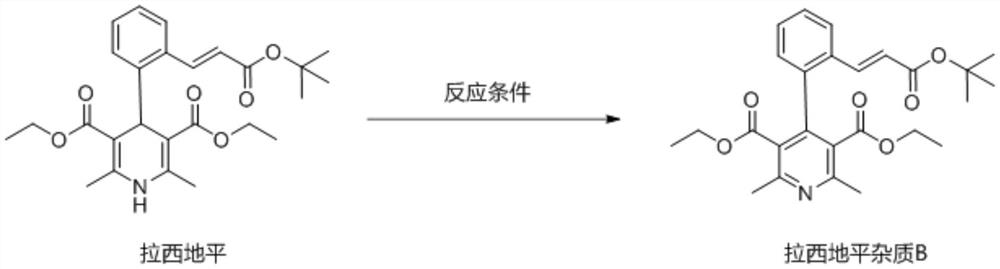
Preparation method of lacidipine impurity B
A technique for lacidipine and impurity, applied in the field of preparation of lacidipine impurity B, can solve problems such as difficulty in applicable industrial production, high catalyst price, difficulty in obtaining impurity B, etc., reduced time to reach, short reaction time, and product impurity little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
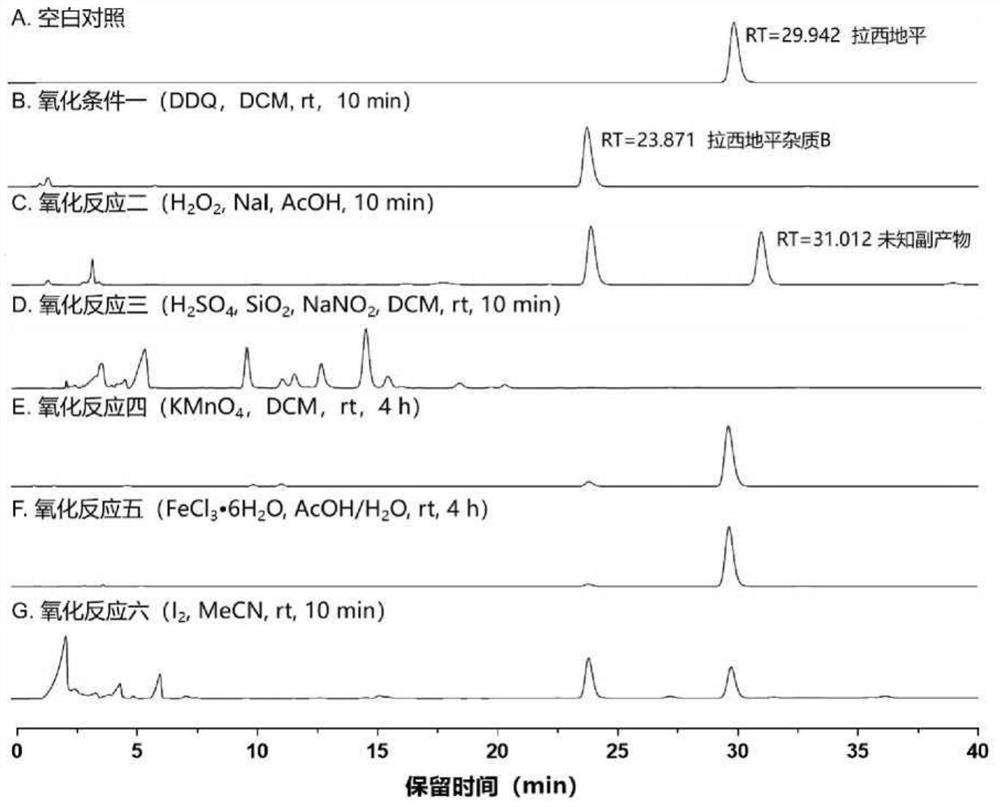
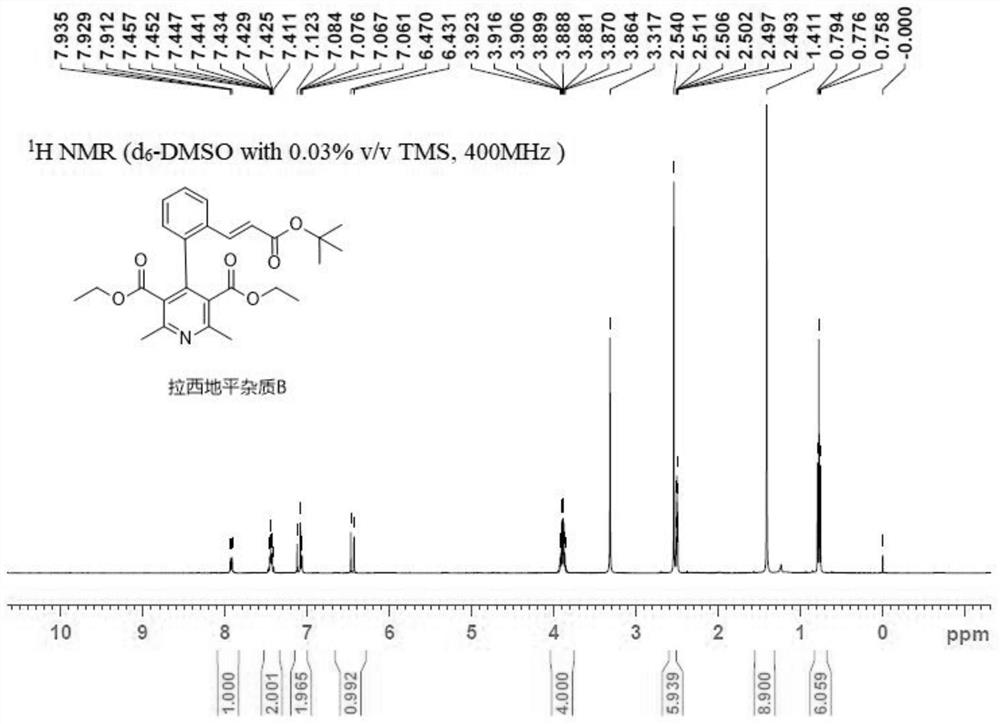
[0042] This embodiment discloses a preparation method of lacidipine impurity B, comprising the following steps:
[0043] Step 1), weigh 2 g of lacidipine in an eggplant-shaped bottle, add 30 mL of dichloromethane and stir until dissolved with a glass rod to obtain a preparatory solution;
[0044] Step 2), add 3gDDQ to the eggplant-shaped bottle at a speed of 1.0g / min, slowly add DDQ to the preparatory solution, stir with a magnetic stirrer at a stirring speed of 250rpm, filter after stirring at room temperature for 10 minutes, and obtain the organic Phase is lacidipine impurity B solution;
[0045] Step 3), add 60mL of 5% sodium bicarbonate aqueous solution to the eggplant-shaped bottle that lacidipine impurity B solution is housed, rinse lacidipine impurity B solution, then add 60mL saturated sodium chloride aqueous solution to rinse, then by placing Spin dry in rotary evaporator, obtain the lacidipine impurity B crude product that is yellow solid;
Embodiment 2
[0047] This embodiment discloses a preparation method of lacidipine impurity B, comprising the following steps:
[0048] Step 1), weigh 10 g of lacidipine in an eggplant-shaped bottle, add 50 mL of acetone and stir until dissolved with a glass rod to obtain a preparatory solution;
[0049] Step 2), add 6gDDQ to the eggplant-shaped bottle at a speed of 1.8g / min, slowly add DDQ to the preparatory solution, stir with a magnetic stirrer at a stirring speed of 300rpm, and filter after stirring for 12 minutes at 20°C to obtain The organic phase is lacidipine impurity B solution;
[0050] Step 3), add 100mL of 15% sodium bicarbonate aqueous solution to the eggplant-shaped bottle that lacidipine impurity B solution is housed, rinse lacidipine impurity B solution, then add 100mL saturated sodium chloride aqueous solution to rinse, then by placing Spin dry in rotary evaporator, obtain the lacidipine impurity B crude product that is yellow solid;
[0051] Step 4), add 20mL of absolute et...
Embodiment 3
[0053] This embodiment discloses a preparation method of lacidipine impurity B, comprising the following steps:
[0054] Step 1), weigh 20g of lacidipine into an eggplant-shaped bottle, add 60mL of dichloromethane, and ultrasonically dissolve for 1 minute to obtain a preparatory solution;
[0055] Step 2), add 30gDDQ to the eggplant-shaped bottle at a speed of 2.2g / min, slowly add DDQ to the preparatory solution, stir with a magnetic stirrer at a stirring speed of 350rpm, and filter after stirring for 13 minutes at 50°C to obtain The organic phase is lacidipine impurity B solution;
[0056] Step 3), add 80mL of 20% sodium bicarbonate aqueous solution to the eggplant-shaped bottle that lacidipine impurity B solution is housed, rinse lacidipine impurity B solution, then add 80mL saturated sodium chloride aqueous solution to rinse, then by placing Spin dry in rotary evaporator, obtain the lacidipine impurity B crude product that is yellow solid;
[0057] Step 4), add 30mL of ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com