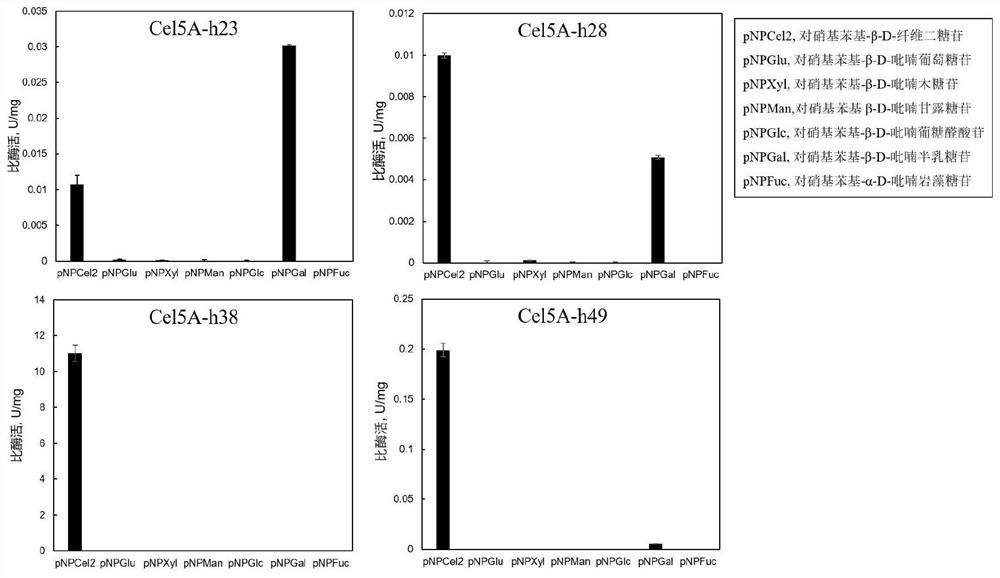
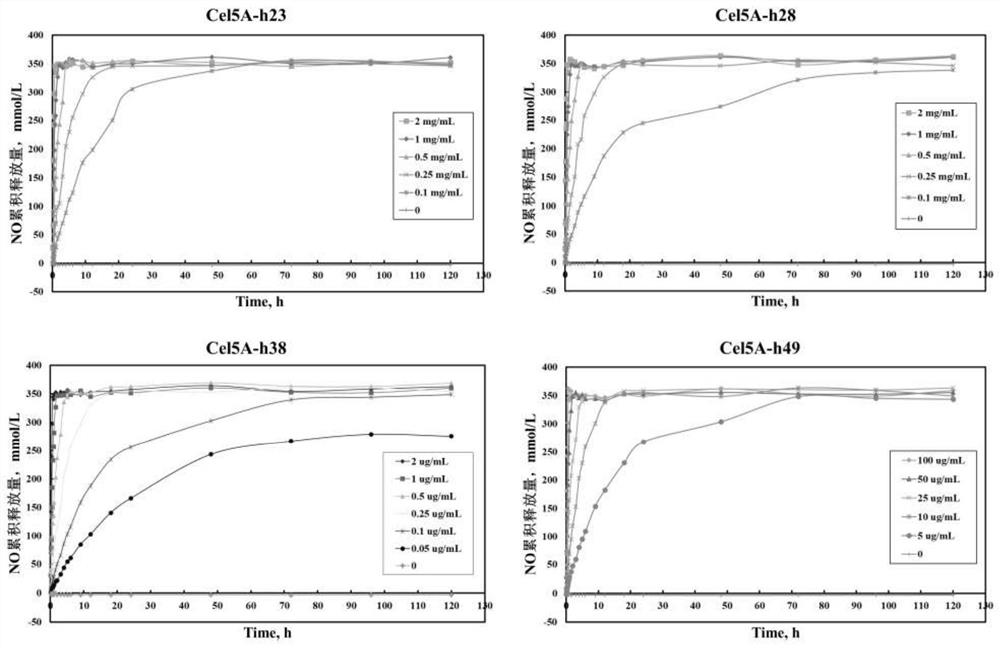
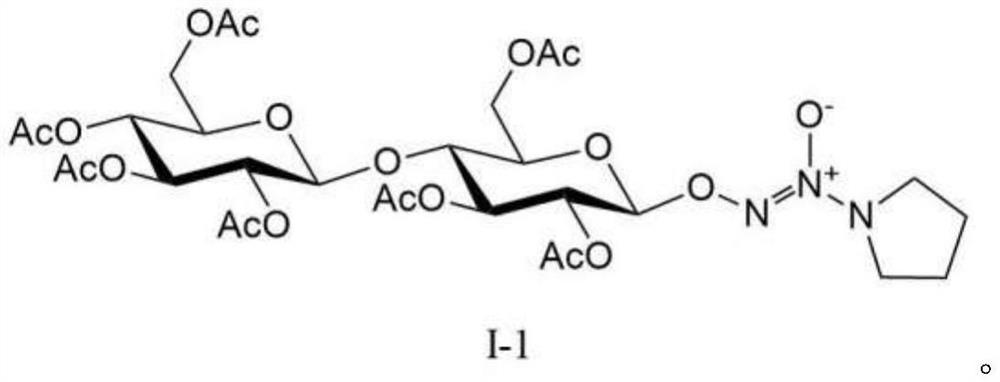
Compound, nitric oxide donor prodrug compound as well as preparation method and application of compound and nitric oxide donor prodrug compound
A technology of nitric oxide and compounds, applied in the field of nitric oxide donor prodrug compounds and their preparation, can solve the problems of non-specific hydrolysis of endogenous glycosides, unpredictable physiological consequences, blood pressure drop, etc., to improve the target tropism, avoid non-specific degradation, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] 1. Synthesis of azoenol donor compound (Cel2-NONOate) with cellobioside modification
[0057] (1) Weigh cellobiose (5.00g, 14.6mmol) in a 100mL three-necked flask, under nitrogen protection, add anhydrous pyridine (30mL), use a constant pressure dropping funnel, add dropwise acetic anhydride ( 18 mL), after the addition was complete, the ice bath was removed and stirred at room temperature for 12 h. The reactant disappears. Spin the solvent with an oil pump, add 100mL DCM dichloromethane to dilute, then wash with saturated CuSO 4 (100mL×2), washed with saturated brine (100mL). Anhydrous Na for organic phase 2 SO 4 Dry, filter and spin the filtrate. 7.0 g of white solid powder of fully acetylated cellobiose was obtained, with a yield of 70%.
[0058] (2) Weigh fully acetylated cellobiose (7.0 g, 10.3 mmol) and dissolve it in acetic anhydride (7 mL). 33% HBr / AcOH solution (17.5 mL) was slowly added dropwise with stirring in an ice bath, shielded from light, the ice...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap