Nitazoxanide derivative and medical application thereof
A technology of nitazoxanide and derivatives is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, applications, etc., and can solve problems such as restricting new medicinal uses and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
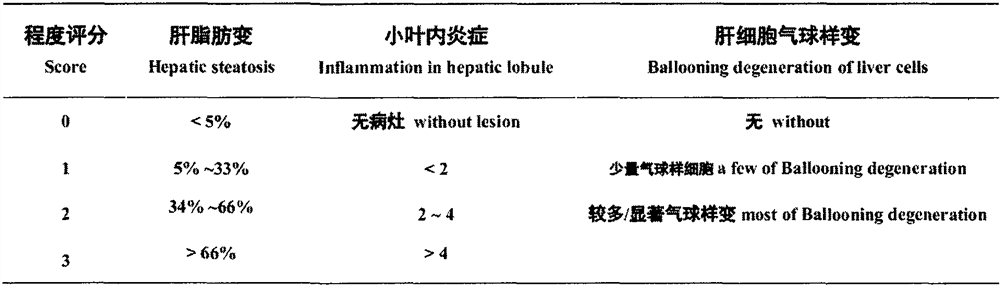
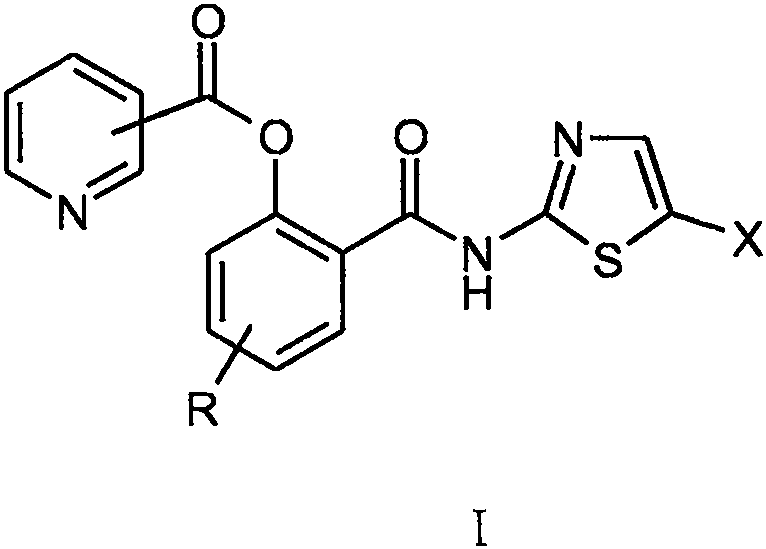
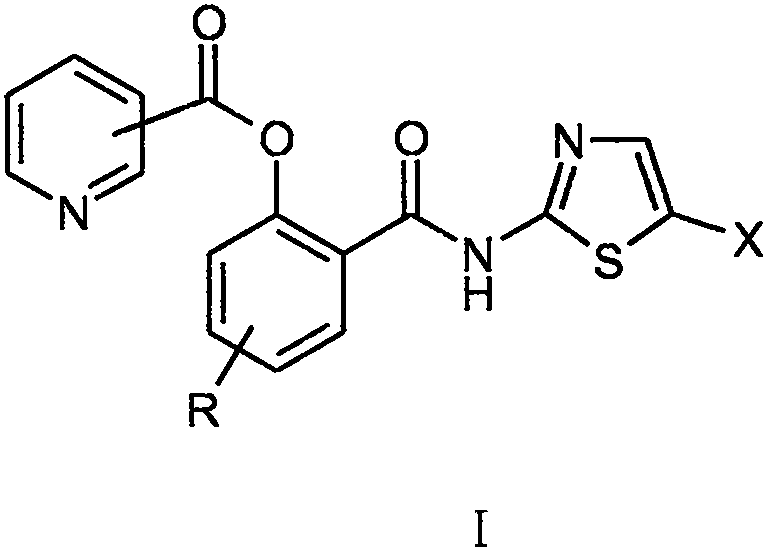
[0028] Example 1 Preparation of o-N-(5-nitrothiazol-2-yl)carbamoyl-phenol-pyridine-3-carboxylate (I-1)
[0029]
Embodiment 11
[0030] Embodiment 1.1 The synthesis of deacetyl nitazoxanide
[0031] Take 30.00 g of nitazoxanide, add it to a 250 ml single-necked bottle, add 180 ml of concentrated hydrochloric acid, stir and disperse at room temperature; heat the system to 50°C and stir for about 15 hours. After completion of the reaction, the reaction system was cooled to room temperature, filtered, and the filter cake was washed with 50 ml × 3 times of purified water. After pumping to dryness, the filter cake was air-dried at 60° C. for 12 hours to obtain 21.1 g of deacetyl nitazoxanide as a pale yellow solid.
Embodiment 12
[0032] Example 1.2 Synthesis of o-N-(5-nitrothiazol-2-yl)carbamoyl-phenol-pyridine-3-carboxylate (I-1)
[0033] Under the protection of argon, add 3.0 g of deacetyl nitazoxanide to 75 ml of tetrahydrofuran solvent, add 5.6 g of triethylamine under stirring, stir and dissolve until clear; cool in an ice bath to 0°C, add 5.8 g of it in batches under stirring Pyridine-3-formyl chloride hydrochloride, after the addition was completed, it was stirred for 0.5 hours under the condition of ice bath and under the protection of argon, and then left at room temperature for 15 hours to react. The insolubles were removed by filtration, the filter cake was washed with 5ml of tetrahydrofuran, and the washed filtrates were combined and evaporated to dryness under reduced pressure. The residue was separated by silica gel column chromatography and eluted with dichloromethane / methanol (10 / 1). The desired components were collected and evaporated to dryness under reduced pressure to obtain 2.3 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com