CHO-DHFR <+> cell strain and application thereof
A technology of CHO-DHFR and cell lines, which is applied in the direction of animal cells, reproductive tract cells, interleukin, etc., can solve the problems of increasing the workload of screening cell clones, degradation products that are unfavorable for cell growth, and high cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: CHO-DHFR+ cell line screening process
[0046] This embodiment provides a CHO-DHFR+ cell line, the CHO-DHFR+ cell line is obtained by screening by the following method:
[0047] (1) Resuscitate CHO-DG44 cells (obtained from the market) for subculture adaptation, and prepare for transfection when the cell growth status returns to normal.
[0048] (2) On the day of transfection, CHO-DG44 cells were washed by OptiMEM centrifugation and resuspended to 3.0×10 6 cells / ml, and then add the cells to a 6-well plate at 0.8ml / well for later use.
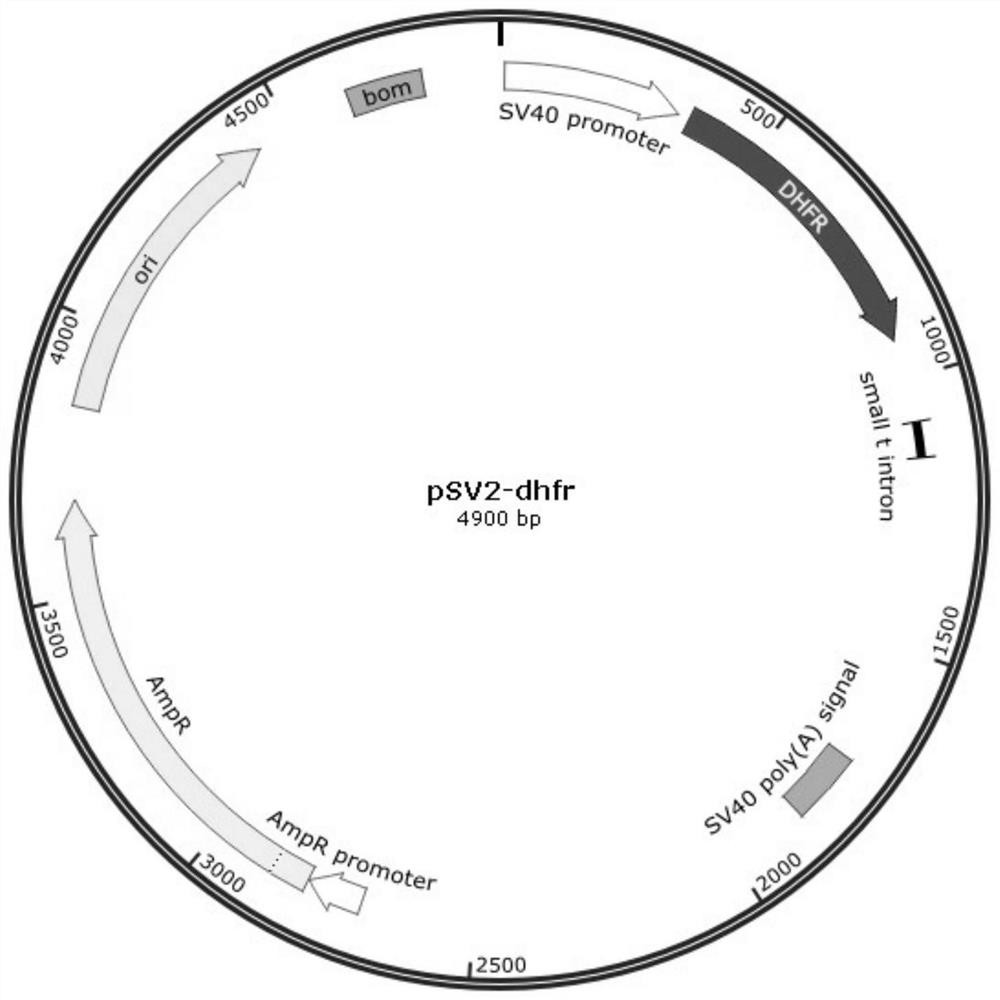
[0049] (3) Mix DNA (per well), add 2.5 μg of DNA (pSV2-DHFR plasmid) into 100 μL of OptiMEM medium, and mix gently. For the blank wells, 2.5 μL of PBS was used instead of DNA, and the mixture was incubated at room temperature for 30 min.
[0050] The information of the pSV2-DHFR plasmid is shown in Table 1 below:
[0051] Table 1:
[0052]
[0053]
[0054] The nucleotide sequence of the pSV2-DHFR plasmid is shown i...
Embodiment 2
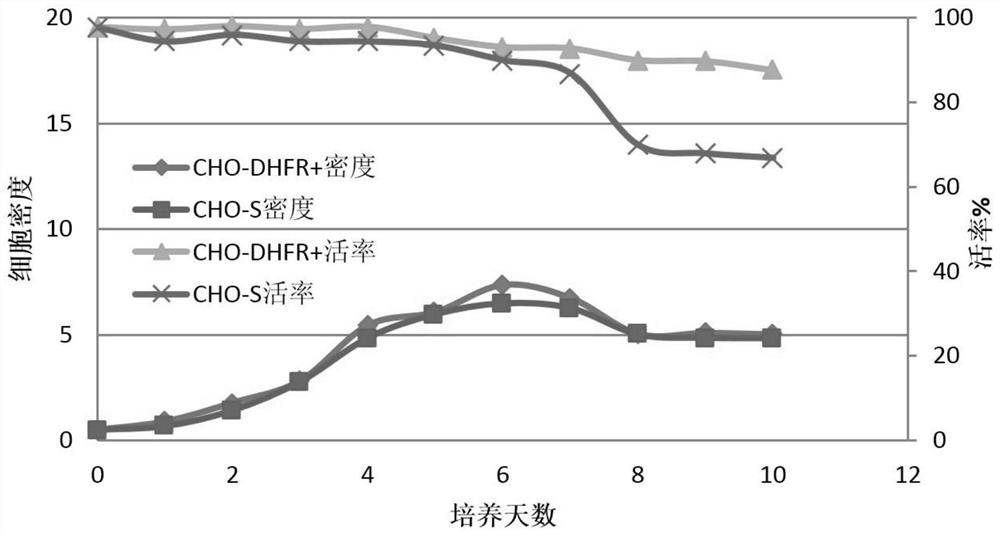
[0069] Example 2: Comparative evaluation of the growth performance of CHO-DHFR+ cell lines
[0070] The CHO-DHFR+ cell line constructed and preserved in Example 1 of the present invention and the commercially available CHO-S cell line were subjected to a comparison experiment of cell growth performance evaluation, as follows:
[0071] (1) resuscitating the CHO-DHFR+ cell line of Example 1 of the present invention and the commercially available CHO-S cell line (control group) in fresh CD OptiCHO medium (containing 3mM glutamine) for subculture adaptation, to the cell The growth status returned to normal.
[0072] (2) Cells recovered to normal growth state were treated with 0.5×10 6 The cells / ml density was inoculated into 125ml shake flasks, 30ml per bottle.
[0073] (3) The day of inoculation is recorded as day0, and 50 μl of cell suspension is taken every day after inoculation to test the cell viability and density on a cell counter, and the test data of day1, day2, day3......
Embodiment 3
[0076] Example 3: Construction of engineered cell lines highly expressing recombinant IL-10
[0077] This embodiment provides a method for constructing an engineered cell line that highly expresses recombinant IL-10, which includes the following steps:
[0078] (1) Resuscitate the CHO-DHFR+ cell line of Example 1 of the present invention into fresh CD OptiCHO medium for subculture adaptation until the cell growth state returns to normal.
[0079] (2) Cells recovered to normal growth state were treated with 0.5×10 6 The cells / ml density was inoculated into 125ml shake flasks, 30ml per bottle.
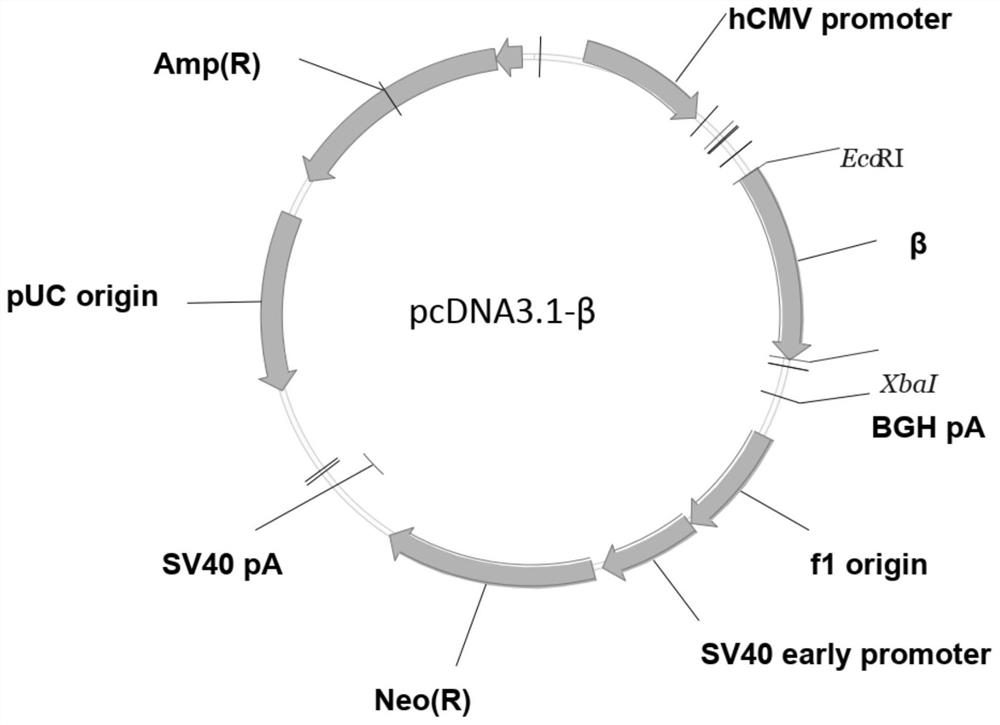
[0080] (3) Link the optimized IL-10 gene (its nucleotide sequence as shown in SEQ ID NO: 1) to the pcDNA3.1-beta plasmid to construct and obtain the recombinant plasmid vector (its nucleotide sequence as shown in SEQ ID NO: 1) NO: 7).
[0081] The pcDNA3.1-β plasmid is as follows:
[0082] The replicon of the pcDNA3.1-β plasmid is pUC origin, the promoter is CMV promoter, the resista...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com