Method for biosynthesizing D-allose by utilizing D-glucose
A technology of biosynthesis and allose, applied in the direction of microorganism-based methods, biochemical equipment and methods, botany equipment and methods, etc., to achieve the effect of increasing yield and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0028] The types of culture medium are as follows
[0029] LB medium:
[0030] Peptone 10g / L
[0032] Sodium chloride 10g / L
[0033] M9 medium:
[0034]
[0035] The bacterial strain JM109(DE3)-pETDuet-1-dpe-rpiB-gi-pRSFDuet-1-Galp of the synthetic D-allose that will be constructed, (plasmid construction such as figure 1 ) stored at -80°C, take it out and place it at room temperature, take 4 microliters into a 4 ml sterilized LB test tube in an ultra-clean bench, and add ampicillin and kanamycin to one-thousandth of the volume of the test tube culture medium, Cultivate overnight for 14-16h.
[0036]Then, inoculate the above-mentioned bacteria into the shake flask LB medium with one thousandth of ampicillin and kanamycin, the inoculation amount is about one thousandth of the culture volume. , cultured for 48-72 hours, the OD600 of the control group JM109(DE3)-pETDuet-1-pRSFDuet-1 did not change from the experimental group, and the production ...
Embodiment example 2
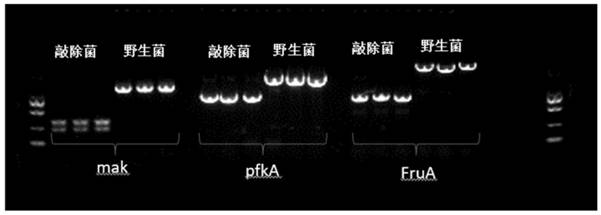
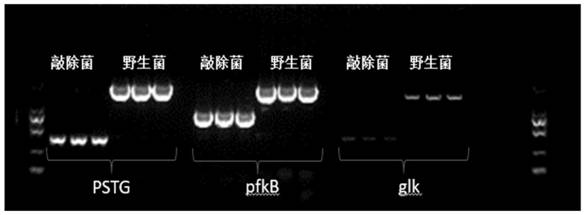
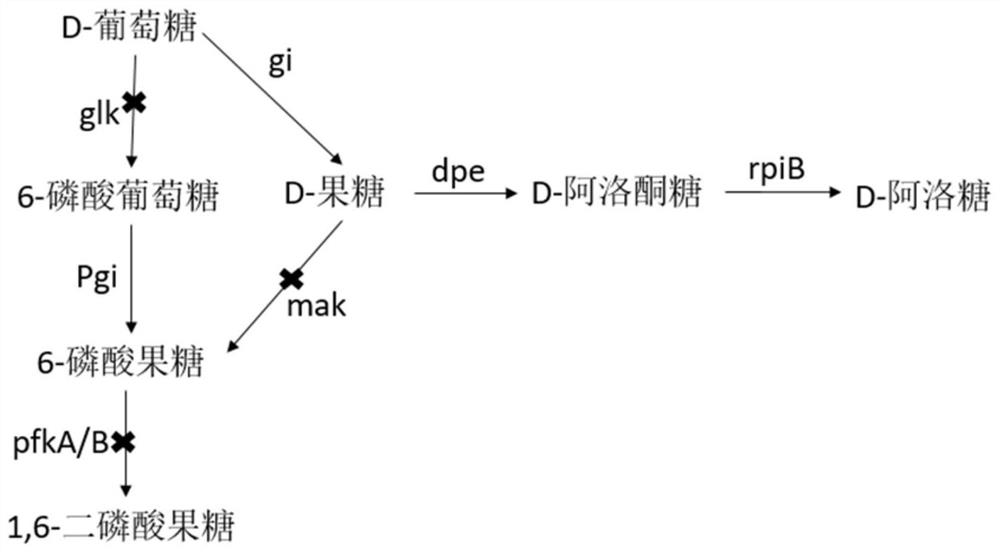
[0038] On the engineering bacteria chassis cell JM109(DE3), the pathway modification was carried out, and D-fructose was an important intermediate product, and because of its position in the glycolysis pathway, when paying attention to the process of the D-glucose metabolism pathway, it was also Pay attention to the metabolic pathway of D-fructose. Therefore, the order of knockout genes must be FruA, ptsG, and the ccr effect can be removed after knockout, so that the co-utilization of glucose and glycerol can be realized. Then knock out pfka, glk, mak, pfkb. To block the consumption of glucose and fructose. Knockout of genes involved in metabolic pathways. Red homologous recombination is used to knock out FruA and ptsG (the phosphorylation pathway for D-glucose and D-fructose to enter the cell). The purpose of knocking out these two genes is to relieve the ccr effect and improve the efficiency of the non-phosphorylation pathway .
[0039] The primers for gene knockout are ...
Embodiment example 3
[0043] First prepare the bacterial strain carrying the corresponding plasmid, insert 4 microliters of the bacterial strain (JM109DE3 / six-knockout JM109DE3) into an LB test tube, and cultivate overnight at 37°180rmp; amplify, take 20 microliters of seed liquid and insert it into a small tube containing 20 mL of LB medium In shake flasks, culture for two hours, OD is about 0.6-0.8; take out shake flasks and ice bath for 10 minutes to ensure cell viability; aliquot, each tube 1mL into 16 1.5mL centrifuge tubes, centrifuge at 6000rmp 4℃ 4min, discard the supernatant; combine the tubes, suspend the precipitation with 800 microliters of 10% glycerol, 4 tubes are gradually combined into 1 tube, and then centrifuge at 6000rmp 4°C for 4min, discard the supernatant; wash bacteria, use 800 microliters of each tube After suspension with 10% glycerol, centrifuge at 6000rmp for 4 minutes at 4°C, discard the supernatant; repeat the previous step; the last four tubes are suspended with 100 mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com