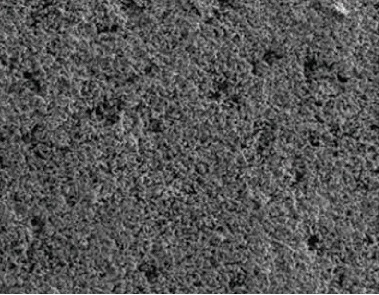
Long-life copper-based composite material
A copper-based composite material, high-life technology, applied in the field of electrochemistry, can solve the problems of unresearched electrocatalytic electrode life, catalytic activity needs to be improved, etc., to achieve the effect of improving contact specific surface area, high ethylene selectivity, and avoiding binding reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A long-life copper-based composite material, prepared by the following method:
[0058] (1) Pretreating the copper-based alloy; the copper-based alloy is copper-nickel-nickel alloy.
[0059] Among them, the pretreatment of step (1) is grinding and inorganic degreasing: the grinding is: use 200#, 600#, 1200# sandpaper for sanding in sequence.
[0060] Degreasing is 15g / L Na 2 CO 3 , 10g / L Na 3 PO 4 . 12H 2 O, 10 g / L Na 2 SiO 3 , the temperature is 50 o C, time 2min.
[0061] (2) The copper-containing solution is used as the electrolyte, and the copper-based alloy is used as the cathode, and the electrochemical deposition reduction is carried out at a very high negative bias voltage.
[0062] Wherein the copper-containing electrolyte in step (2) includes copper sulfate, sulfuric acid, chloride ions and surfactants, the anode is an inert electrode or pure copper, and the copper-containing electrolyte: 140g / L CuSO 4 . 5H 2 O; 30g / L H 2 SO 4 ; 40g / L Cl - ;0.5...
Embodiment 2
[0072] A long-life copper-based composite material, prepared by the following method:
[0073] (1) Pretreating the copper-based alloy; the copper-based alloy is copper-nickel-nickel alloy.
[0074] Among them, the pretreatment of step (1) is grinding and inorganic degreasing: the grinding is: use 200#, 600#, 1200# sandpaper for sanding in sequence.
[0075] Degreasing is 15g / L Na 2 CO 3 , 10g / L Na 3 PO 4 . 12H 2 O, 10 g / L Na 2 SiO 3 , the temperature is 50 o C, time 2min.
[0076] (2) The copper-containing solution is used as the electrolyte, and the copper-based alloy is used as the cathode, and the electrochemical deposition reduction is carried out at a very high negative bias voltage.
[0077] Wherein the copper-containing electrolyte in step (2) includes copper sulfate, sulfuric acid, chloride ions and surfactants, the anode is an inert electrode or pure copper, and the copper-containing electrolyte: 145g / L CuSO 4 . 5H 2 O; 32.5g / L H 2 SO 4 ; 42.5g / L Cl - ...
Embodiment 3
[0087] A long-life copper-based composite material, prepared by the following method:
[0088] (1) Pretreating the copper-based alloy; the copper-based alloy is copper-nickel-nickel alloy.
[0089] Among them, the pretreatment of step (1) is grinding and inorganic degreasing: the grinding is: use 200#, 600#, 1200# sandpaper for sanding in sequence.
[0090] Degreasing is 15g / L Na 2 CO 3 , 10g / L Na 3 PO 4 . 12H 2 O, 10 g / L Na 2 SiO 3 , the temperature is 50 o C, time 2min.
[0091] (2) The copper-containing solution is used as the electrolyte, and the copper-based alloy is used as the cathode, and the electrochemical deposition reduction is carried out at a very high negative bias voltage.
[0092] The copper-containing electrolyte in step (2) includes copper sulfate, sulfuric acid, chloride ions and surfactants, the anode is an inert electrode or pure copper, and the copper-containing electrolyte: 150g / L CuSO 4 . 5H 2 O; 35g / L H 2 SO 4 ; 45g / L Cl - ; 1g / LAPEO; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com