Phloroglucinol injection and preparation method thereof
A phloroglucinol injection, phloroglucinol technology, applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, drug delivery, etc., can solve the problems of rapid growth of substances, easy oxidation and instability of phloroglucinol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
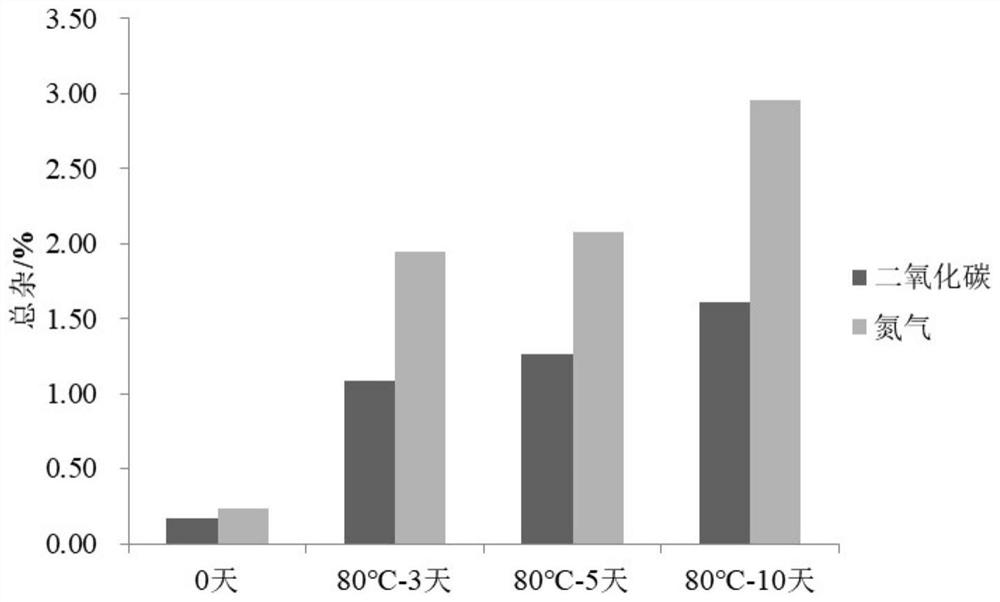
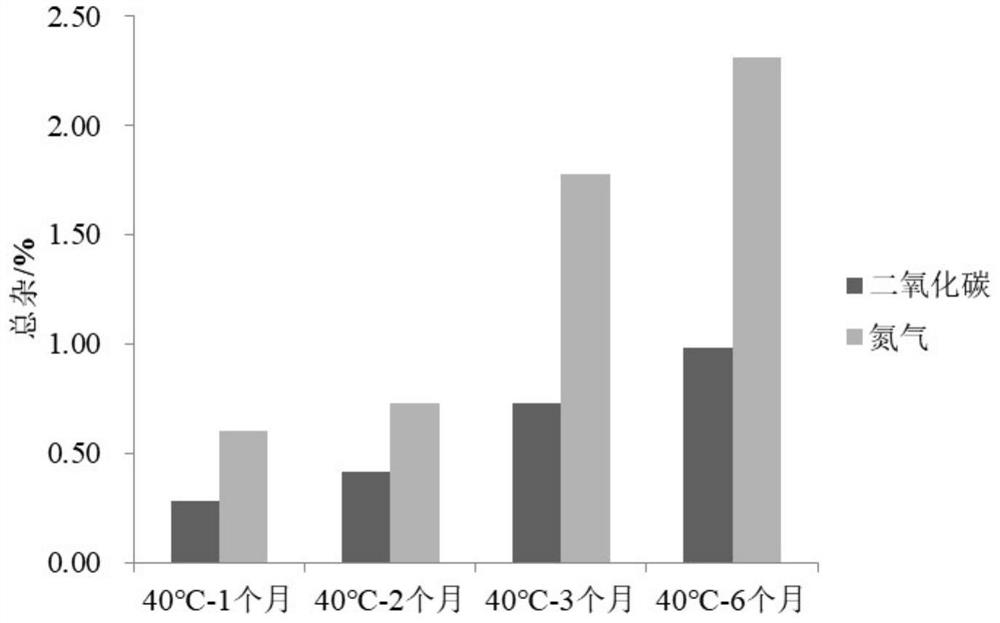
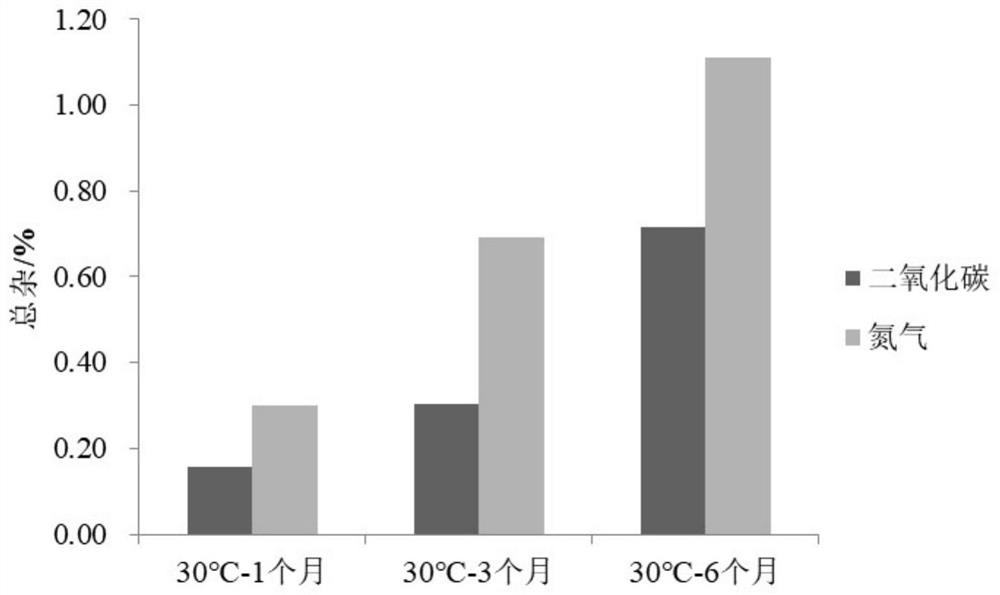
[0038] Embodiment 1 prepares phloroglucinol injection (contrasting the impact of feeding gas as carbon dioxide or nitrogen on the stability of the preparation)
[0039] Single bottle dose: total volume: 4mL / bottle, phloroglucinol hydrate: 40mg / bottle, the prescription is as follows:
[0040] Phloroglucinol Hydrate 40mg
[0041] Trimethylphloroglucinol 0.04mg
[0043] Hydrochloric acid amount
[0044] Appropriate amount of water for injection
[0045] Gas Carbon Dioxide / Nitrogen
[0046] Example 1 Preparation process: add 80% of the prescribed amount of water for injection at 80°C, and simultaneously feed carbon dioxide / nitrogen to make it saturated in water. Add the prescribed amount of trimethylphloroglucinol, phloroglucinol hydrate and sodium chloride and stir for 5 minutes, adjust the pH to 4.00 with hydrochloric acid, add water for injection to a sufficient amount and stir for 10 minutes, pass the solution through a 0.22 μm filter membran...
Embodiment 2
[0057] Example 2 Preparation of Phloroglucinol Injection (Contrast the Effect of Deoxygenation on Preparation Stability)
[0058] Single bottle dose: total volume: 4mL / bottle, phloroglucinol hydrate: 40mg / bottle, the prescription is as follows:
[0059] Phloroglucinol Hydrate 40mg
[0060] Trimethylphloroglucinol 0.04mg
[0061] Sodium chloride 28mg
[0062] Hydrochloric acid amount
[0063] Appropriate amount of water for injection
[0064] Gas CO2 / None
[0065] Example 2 Preparation process: add 80% of the prescribed amount of water for injection at 80°C, and simultaneously feed carbon dioxide or not feed gas. Add the prescribed amount of trimethylphloroglucinol, phloroglucinol hydrate, and sodium chloride, stir for 5 minutes, stir to dissolve completely, adjust the pH of the solution to 4.00 with hydrochloric acid, supplement water for injection to a sufficient amount, and add 0.22 Filter with μm filter membrane and potting.
[0066] Investigate the related substances ...
Embodiment 3
[0070] Example 3 Preparation of Phloroglucinol Injection (Contrasting the Impact of Antioxidants on Preparation Stability)
[0071] Single bottle dose: total volume: 4mL / bottle, phloroglucinol hydrate: 40mg / bottle, the prescription is as follows:
[0072] Phloroglucinol Hydrate 40mg
[0073] Trimethylphloroglucinol 0.04mg
[0074] Sodium chloride 28mg
[0075] Antioxidant (sodium bisulfite / sodium metabisulfite) 8mg
[0076] Hydrochloric acid amount
[0077] Appropriate amount of water for injection
[0078] gas nitrogen
[0079] Example 3 Preparation process: add 80% of the prescription water for injection at 80° C., and feed nitrogen at the same time. Add the prescribed amount of antioxidant (sodium bisulfite / sodium pyrosulfite), trimethylphloroglucinol, phloroglucinol hydrate, and sodium chloride, stir for 5 minutes, stir to dissolve completely, and adjust the solution with hydrochloric acid pH to 4.00, supplemented with water for injection to a sufficient amount, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com