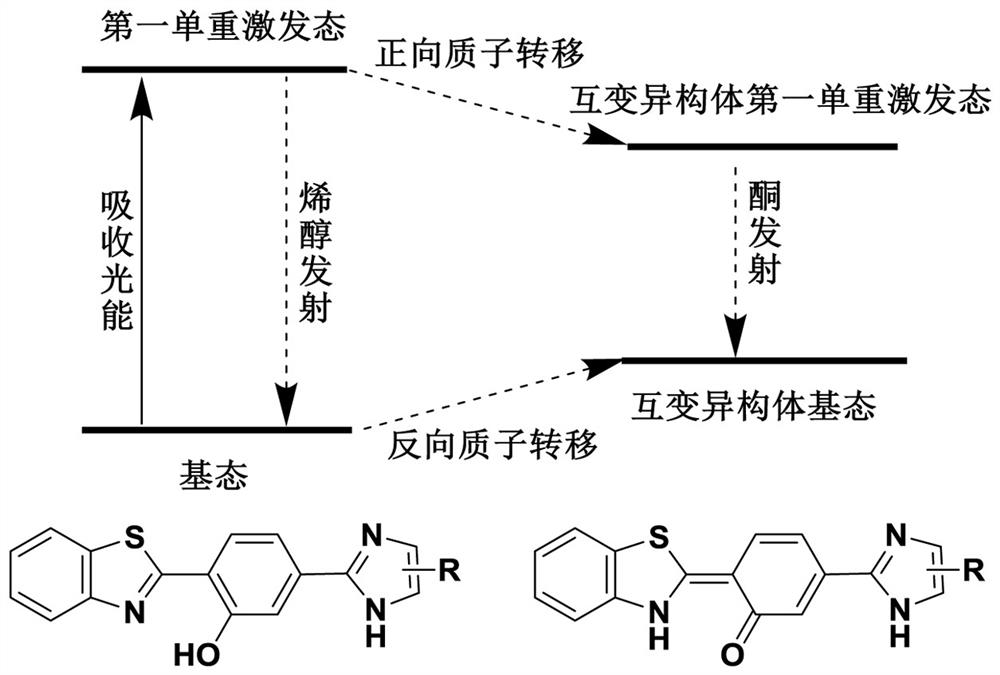
Benzothiazole derivative and application thereof as fluorescent dye
A technology of benzothiazole and fluorescent dyes, applied in the directions of luminescent materials, azo dyes, organic dyes, etc., can solve problems such as the inability to meet the needs of analysis and detection, and achieve the effects of easier products, mild reaction conditions, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
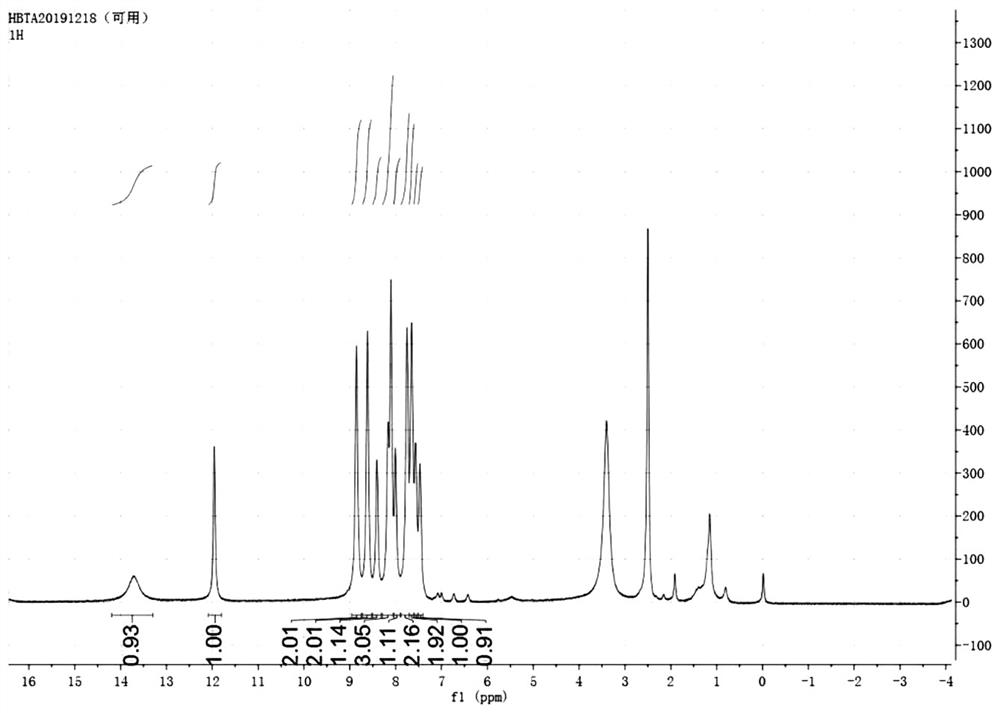
[0050] Example 1: Synthesis of Compound HBTA
[0051]It is weighing 4- (benzo [D] thiazole-2-yl) -3-hydroxybenzaldehyde (63.1 mg, 0.25 mmol), 9,10-phenanthin (63.1 mg, 0.30 mmol), ammonium acetate (95.3 mg, 1.24 mmol) was dissolved in 5 ml of acetic acid, reflux at 110 ° C, and the reaction TLC dot plate monitored to the feedstock reaction, poured into 20 ml of water, yellow solid precipitation, washed after filtration, and dried to give a yellow solid after drying. That is, the dye HBTA, 52.6 mg (yield 47%).
Embodiment 2
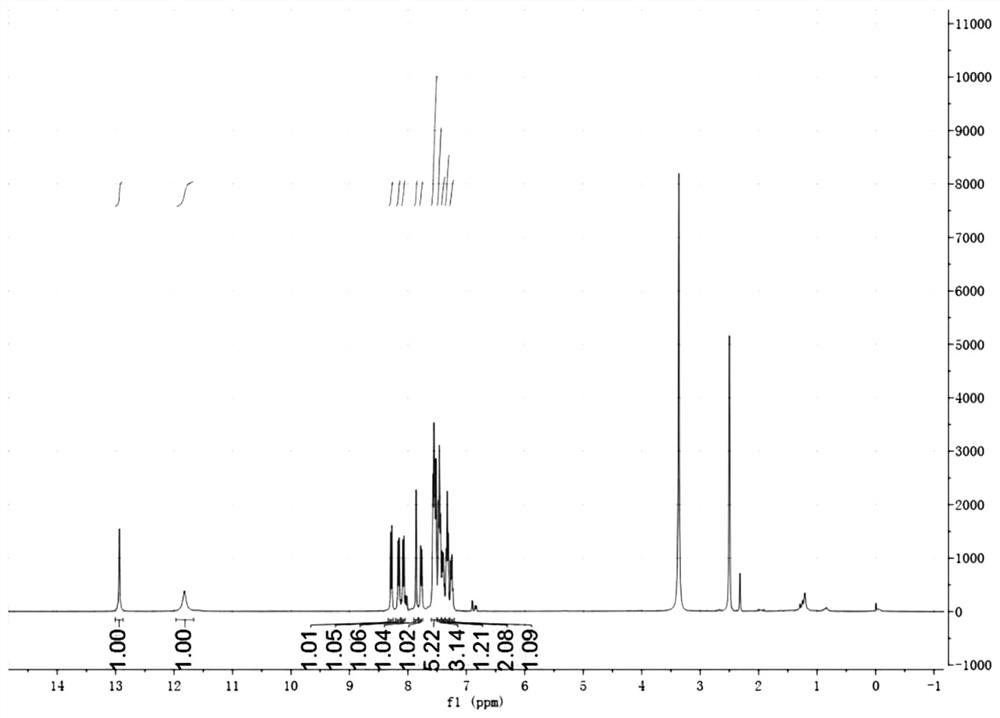
[0052] Example 2: Synthesis of Compound HBTB
[0053] 4- (benzoxyl [D] thiazole-2-yl) -3-hydroxybenzaldehyde (76.3 mg, 0.3 mmol), benzophenyl (108.8 mg, 0.52 mmol), ammonium acetate (123 mg, 1.6 mmol) Dissoluble in 5 ml of acetic acid, refluxed at 110 ° C, and the TLC dot plate monitored to the feedstock reaction. The reaction solution was poured into 20 mL of water, and yellow solid precipitated, washed after filtration, and dried, it gave a pale yellow solid, ie the dye HBTb, 82.8 mg (yield 62%).
Embodiment 3
[0054] Example 3: Synthesis of Compound HBTC
[0055] Tighted 4- (benzo [D] thiazol-2-yl) -3-hydroxybenzaldehyde (63.1 mg, 0.25 mmol), 1,10-Philippine-5,6-dilute (56.4 mg, 0.26 mmol ), Ammonium acetate (59.1 mg, 0.76 mmol) is dissolved in 5 ml of acetic acid, refluxed at 110 ° C, and the TLC point plate monitoring reaction is complete, pour the reaction liquid into 20 ml of water, orange solid precipitation, filtration After washing, then the orange solid was obtained, that is, the dye HBTC, 67.4 mg (yield 72%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com