R-transaminase derived from pseudonocardia ammoxidation and synthesis method of R-transaminase
A pseudo-Nocardian and synthetic method technology, applied in the field of production of chiral amines and unnatural amino acids, can solve the problems of high energy consumption, high pollution, etc., achieve broad substrate spectrum, simple preparation method, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one: prepare R-transaminase:
[0026] (1) The construction of the R-transaminase PamAT carrier derived from Pseudonocardia ammoxidans:
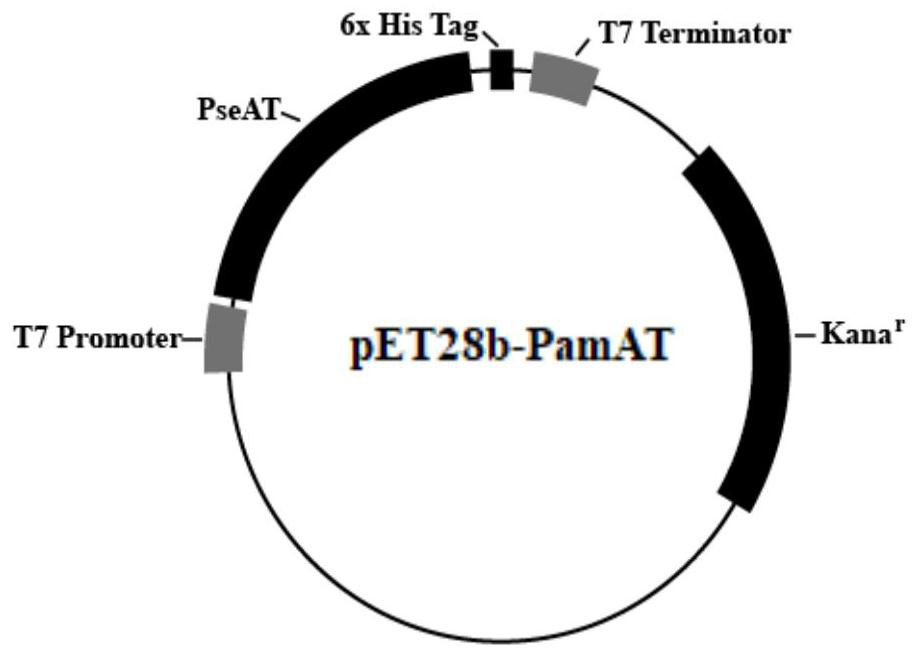
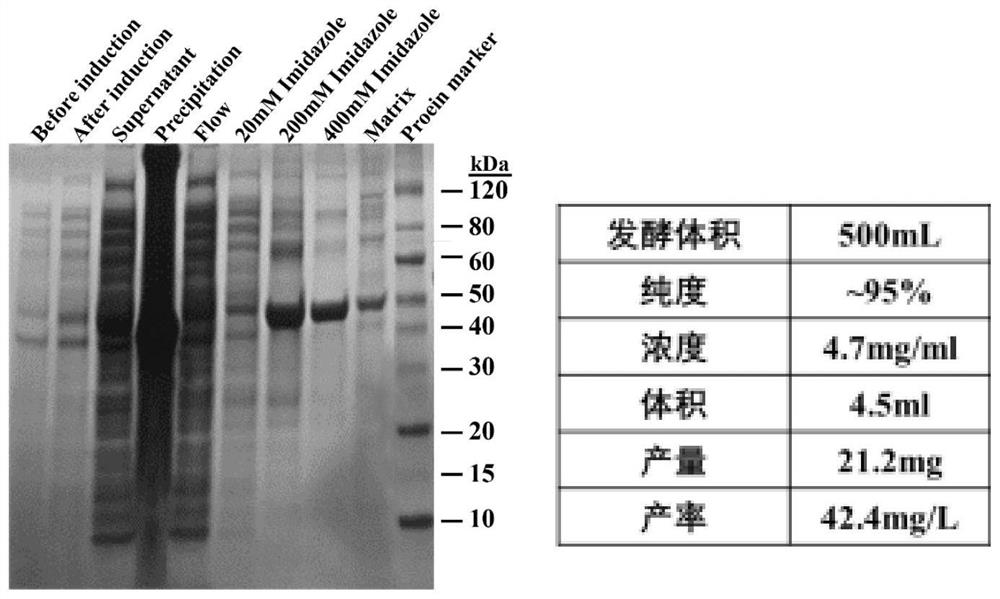
[0027] The R-transaminase PamAT gene sequence of the present invention is derived from the GeneBank database wp-093355841.1. On the premise of not changing the amino acid sequence, the codon of the transaminase gene derived from Pseudomonas ammoniaoxidans is first optimized, and the restriction endonuclease is used to The enzymes Nco I and Hind III were connected (Sangon Bioengineering (Shanghai) Co., Ltd.) to the pET-28b vector, and the plasmid map is as follows figure 1 As shown, the nucleic acid sequence of R-transaminase is shown in SEQ NO.1, and the amino acid sequence is shown in SEQ NO.2. The pET-28b vector is transformed into the competent cells of E. coli DH5α strain, and the shaker is recovered and coated Incubate overnight in an LB culture dish containing kana at a final concentration of 50 μg / ml in an incubator ...
Embodiment 2
[0044] Example 2: PamAT catalyzes donor D-serine and acceptor pyruvate to generate D-alanine and 3-hydroxypyruvate:
[0045] Using 50mmol / L D-serine, 50mmol / L pyruvate, 2mmol / L PLP, and deionized water as raw materials, prepare a transamination reaction solution, adjust the pH to 7.5, take 490μl of the transamination reaction solution, and add 10μl of PamAT enzyme solution , under the condition of 30° C., reacted for 5 h, and measured the amount of the product D-alanine generated by the reacted mixture according to the above method (3).
Embodiment 3
[0046] Example 3: PamAT catalyzes the generation of D-serine from the donor D-alanine and the acceptor 3-hydroxypyruvate:
[0047] Use 50mmol / L D-alanine, 50mmol / L 3-hydroxypyruvate, 2mmol / L PLP, and deionized water as raw materials to prepare a transamination reaction solution, adjust the pH to 7.5, take 490μl of the transamination reaction solution, and add 10μl of The PamAT enzyme solution was reacted at 30° C. for 5 h, and the reaction mixture was measured according to the method in (3) above to determine the amount of D-serine produced.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com