Pharmaceutical composition containing diuretic
A composition and drug technology, applied in the field of pharmacy, can solve the problems of increased risk of heart failure, dyspnea, etc., and achieve the effects of convenient medication, increased curative effect, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7-10
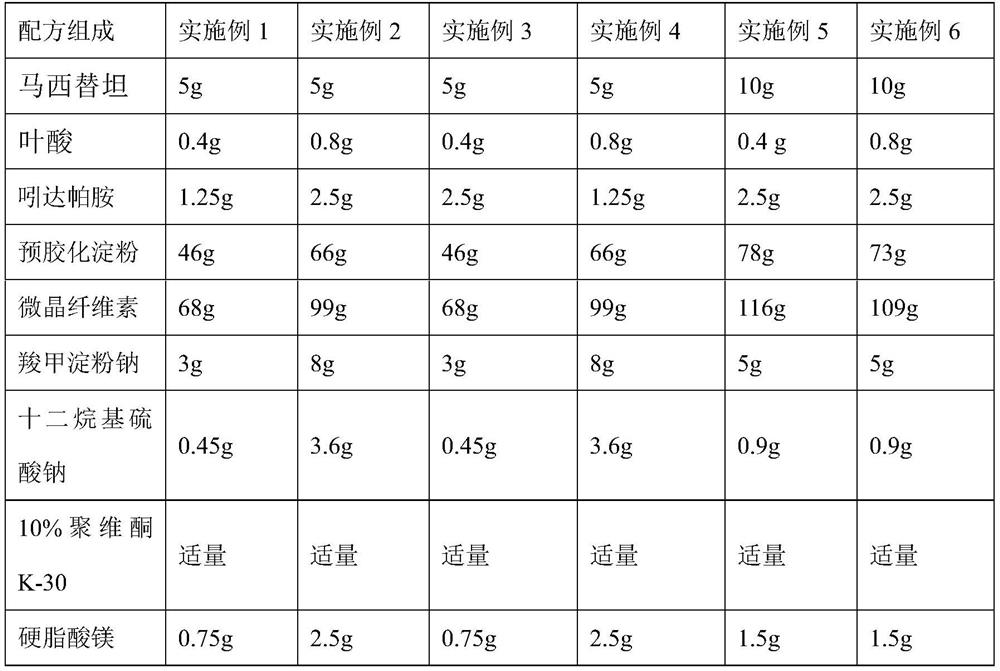
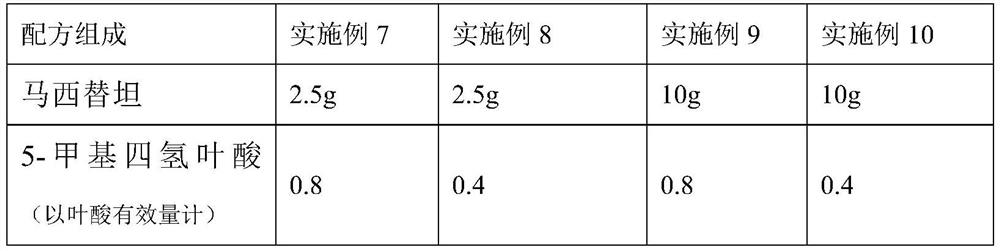
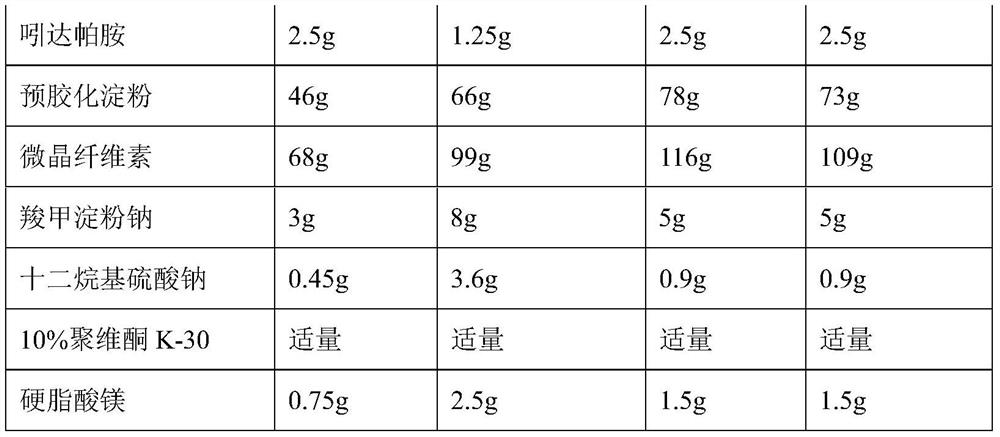
[0031] Embodiment 7-10 prepares macitentan, folic acid and indapamide (1000 tablets)
[0032]
[0033]
[0034] Preparation Process:
[0035] Mix macitentan, folic acid and indapamide, add carboxymethyl starch sodium and sodium lauryl sulfate, then add microcrystalline cellulose and pregelatinized starch and mix evenly, and use an appropriate amount of 10% povidone The ethanol solution is made into a soft material, granulated, dried, and granulated, and the granules with a water content of about 3% are evenly mixed with an appropriate amount of magnesium stearate, and compressed into 1,000 tablets.
Embodiment 11
[0036] Example 11: Efficacy and safety evaluation of macitentan / folic acid / indapamide in the treatment of rats with pulmonary arterial hypertension
[0037] (1) Method
[0038] Experimental modeling: 70 male SD rats were adaptively fed for one week, and 10 rats without any treatment were used as the normal control group. The rest of the rats were given intraperitoneal injection of 0.5ml monocrotaline (MCT) 60mg / kg once, two weeks later the rats with pulmonary hypertension were formed, and then they were randomly divided into groups.
[0039] Experimental grouping and dosing regimen: normal control group (n=10), the rats after modeling were randomly divided into model group and drug administration group, the specific grouping and dosage are shown in Table 1, each group after 4 weeks of intragastric administration After fasting overnight, the hemodynamics of each group were measured 48 hours after drug withdrawal, including right atrial pressure and mean pulmonary artery pressu...
Embodiment 12
[0050] Example 12: Clinical trials of macitentan / folic acid / indapamide in the treatment of pulmonary arterial hypertension
[0051] (1) Method
[0052] Patient source: 43 patients with pulmonary arterial hypertension admitted to the Department of Cardiovascular Medicine of a hospital from January 2015 to January 2017 were used as research objects. All patients underwent pulmonary artery pressure testing through right heart catheterization, all of which conformed to the 2009 American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) jointly issued diagnostic criteria for pulmonary hypertension.
[0053] Patient exclusion criteria: exclude those who meet any of the following:
[0054] ①Patients with various types of congenital heart defects; ②Patients with PAH closely related to factors other than congenital heart disease, such as genetics or HIV; ③Patients with confirmed pulmonary embolism or pulmonary parenchymal diseases; ④Combined with tumors,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap