Synthesis method of ethyl 2,3-dichloro-4-nitrobenzoate
A technology for the synthesis of ethyl nitrobenzoate, which is applied to the preparation of nitro compounds, chemical instruments and methods, and the preparation of organic compounds to achieve the effects of high yield and purity, easy control, and short routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
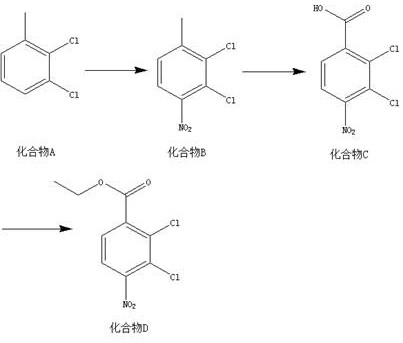
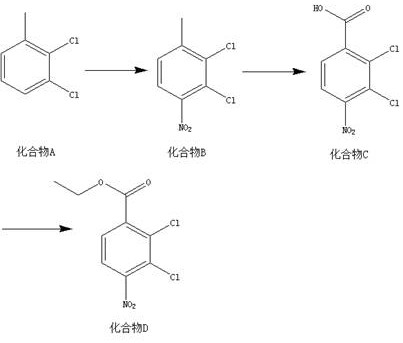
[0018] A kind of synthetic method of 2,3-dichloro-4-nitrobenzoic acid ethyl ester, this synthetic method comprises the steps:
[0019] (1) According to the mass ratio of compound A and nitric acid with a mass fraction of 65% being 10:7~9, the solid-liquid g / mL ratio of compound A and concentrated sulfuric acid is 1:5, and the material is taken, and compound A and concentrated sulfuric acid Add to the reactor, add nitric acid with a mass fraction of 65% dropwise at 0-2°C, raise the temperature to 25-35°C, and react for 24-26 hours to obtain compound B;
[0020] (2) According to the mass ratio of compound B and potassium permanganate is 1:2~5, the ratio of compound B, pyridine and water=g:mL:mL is 1:10:20~25, and the material is taken, and the compound B , pyridine and water are mixed, heated to 85-90°C, potassium permanganate is added, kept warm, and reacted for 23-26 hours to obtain compound C;
[0021] (3) According to compound C, thionyl chloride, ethanol=g:mL:mL ratio of 1...
Embodiment 1
[0023] A kind of synthetic method of 2,3-dichloro-4-nitrobenzoic acid ethyl ester, this synthetic method comprises the steps:
[0024] (1) Add 10g of compound A and 50mL of concentrated sulfuric acid into the reactor, add 7g of nitric acid with a mass fraction of 65% dropwise at 2°C, raise the temperature to 35°C, react for 26 hours, and detect by TLC. After the reaction of the raw materials is complete, slowly pour the reaction solution into Extract with ethyl acetate (800mL*2) in 1000mL ice water, concentrate the organic phase, mix the sample with silica gel, and pass through the column to obtain 12g of yellow solid, namely compound B, with a yield of 93.7% and a purity of 98.2%;
[0025] (2) Mix 1g of compound B, 10mL of pyridine and 20mL of water, heat to 90°C, add potassium permanganate, keep warm, react for 26h, TLC detection, some raw materials are reacted, filtered while hot, washed with hot water, ethyl acetate Extract unreacted raw materials (250mL*2), concentrate th...
Embodiment 2
[0029] A kind of synthetic method of 2,3-dichloro-4-nitrobenzoic acid ethyl ester, this synthetic method comprises the steps:
[0030] (1) Add 10g of compound A and 50mL of concentrated sulfuric acid into the reactor, add 7g of nitric acid with a mass fraction of 65% dropwise at 0°C, raise the temperature to 25°C, react for 24 hours, and detect by TLC. After the reaction of the raw materials is complete, slowly pour the reaction solution into Extract with ethyl acetate (800mL*2) in 1000mL ice water, concentrate the organic phase, mix the sample with silica gel, and pass through the column to obtain 12.1g of yellow solid, which is Compound B, 94.6% with a purity of 97.5%;
[0031] (2) Mix 1g of compound B, 10mL of pyridine and 20mL of water, heat to 85°C, add potassium permanganate, keep warm, react for 23h, TLC detection, some raw materials are reacted, filtered while hot, washed with hot water, ethyl acetate Extract the unreacted raw material (250mL*2), concentrate the water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com