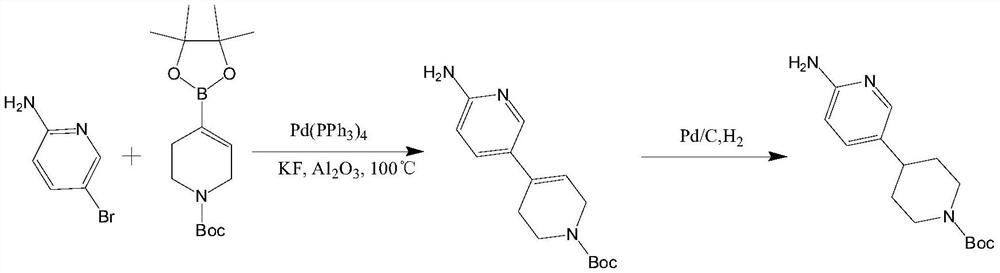
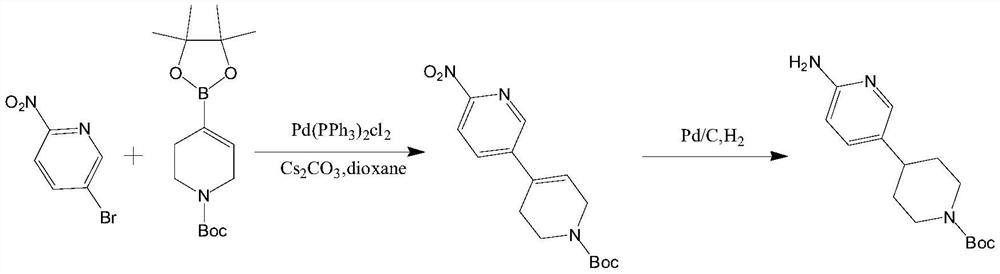
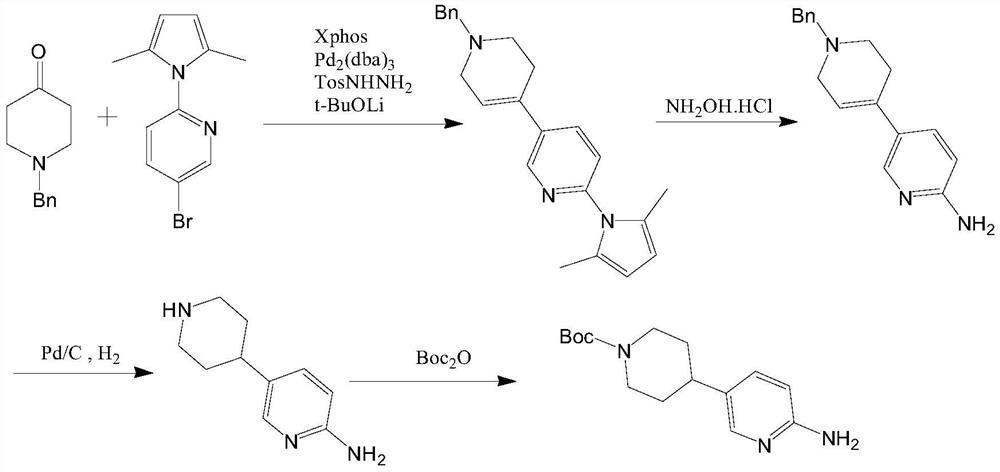
Method for preparing CDK4/6 inhibitor key intermediate through chemical-enzymatic method
An enzymatic preparation and inhibitor technology, applied in organic chemistry, fermentation, etc., can solve the problems of high cost of palladium catalyst and high safety requirements of catalytic hydrogenation equipment, and achieve the effect of short steps, low equipment requirements and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1): Synthesis of tert-butyl 4-(6-nitropyridin-3-yl)piperidine-1-carboxylate
[0038] Under the protection of nitrogen, add 40.6g of 5-bromo-2-nitropyridine into 400ml of anhydrous THF, drop 240ml of tert-butylmagnesium chloride solution (1.0M) at -10~-20°C, and keep the reaction for 2h after dropping Get Grignard reagent. Dissolve 52.8g of N-tert-butoxycarbonyl-4-bromo-piperidine and 324mg of ferric chloride in 500ml of anhydrous THF under the protection of nitrogen, add the above-mentioned Grignard reagent dropwise, control the temperature at -10~-20°C, and keep warm for the reaction 4h, after the completion of the TLC reaction, add saturated ammonium chloride solution, extract with ethyl acetate, dry, evaporate to dryness, add 200ml of methyl tert-butyl ether to reflux for beating for 1h, drop to -5 ~ 0°C, filter with suction, and dry After drying, 55.7 g of the product was obtained, with a yield of 90.7% and a purity of 98.8%.
[0039] 2) Synthesis of tert-butyl 4-...
Embodiment 2
[0042] 1) Synthesis of 4-(6-nitropyridin-3-yl)piperidine-1-carboxylic acid tert-butyl ester
[0043]Under the protection of nitrogen, add 60.9g of 5-bromo-2-nitropyridine into 600ml of anhydrous THF, drop 380ml of tert-butylmagnesium chloride solution (1.0M) at -10~-20°C, and keep the reaction for 2h after dropping Get Grignard reagent. Dissolve 79.2g of N-tert-butoxycarbonyl-4-bromo-piperidine and 486mg of ferric chloride in 750ml of anhydrous THF under the protection of nitrogen, add the above-mentioned Grignard reagent dropwise, control the temperature at -10~-20°C, and keep the reaction 4h, after the TLC reaction is completed, add saturated ammonium chloride solution, extract with ethyl acetate, dry, evaporate to dryness, add 300ml methyl tert-butyl ether to reflux for beating for 1h, drop to -5 ~ 0°C, filter with suction and dry After drying, 83.9 g of the product was obtained, with a yield of 91.1% and a purity of 98.9%.
[0044] 2) Synthesis of tert-butyl 4-(6-aminopy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com