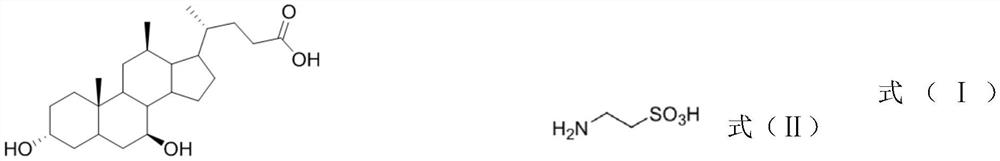Application of silane and synthesis of tauroursodeoxycholic acid under catalysis of silane
A technology of tauroursodeoxycholic acid and ursodeoxycholic acid is applied in the directions of steroids, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc. Environmental protection and other issues, to achieve the effect of low cost, strong tolerance, and simplified synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
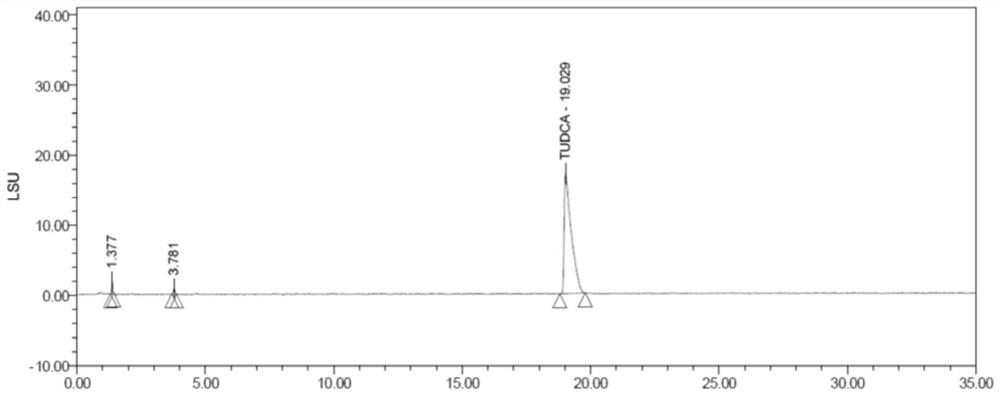
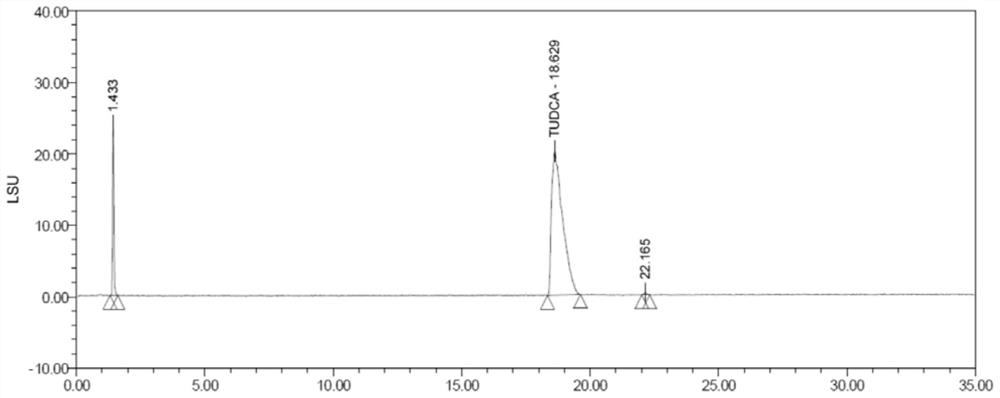
[0026] The structural formulas of ursodeoxycholic acid (cas: 128-13-2) and taurine (cas: 07-35-7) are respectively shown in formula (I) - formula (II), and the molecular formula of hydrosilane is HSi(OCH (CF 3 ) 2 ) 3 , the molecular formula of aminosilane is PMBNHSi(OCH(CF 3 ) 2 ) 3 , the synthesis process of tauroursodeoxycholic acid is shown in formula (Ⅲ).
[0027]
[0028]
[0029] The synthetic method of tauroursodeoxycholic acid under silane catalysis is specifically as follows:
[0030] Synthesis steps of tauroursodeoxycholic acid: 1 mol of ursodeoxycholic acid is dissolved in tetrahydrofuran, wherein the amount of tetrahydrofuran (mL) is 4 times the mass (g) of ursodeoxycholic acid, and the unit is mL. Then, add 1 mol of hydrosilane and 0.03 mol of aminosilane, and finally add 1 mol of taurine, keep stirring at 25°C for 6 hours, and the stirring speed is 180 rpm, and finally obtain system I.
[0031] Purification steps of tauroursodeoxycholic acid: filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com