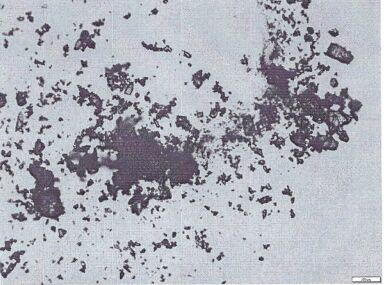
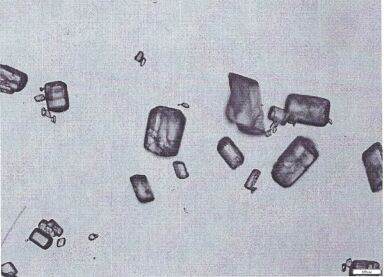
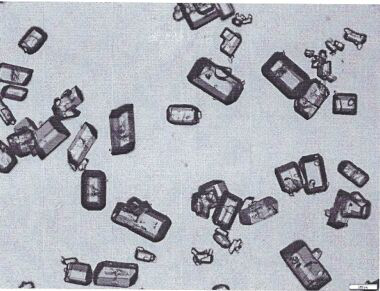
Nifedipine A crystal bulk crystal habit and controlled release tablet composition thereof
A nifedipine and composition technology, applied in the field of nifedipine A crystal block crystal habit and its controlled-release tablet composition, can solve the preparation process of large nifedipine controlled-release tablets, the difficulty of controlling the dissolution rate of preparations, the nifedipine Problems such as the poor crystal form of bendipine can facilitate commercial production, avoid the risk of a narrow process operation window, and solve the effects of large release differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1. Add 4g of nifedipine into a crystallizer filled with 100g of methanol and water mixed solvent (mass ratio: 17:3), stir and dissolve at 40°C, and after continuous stirring for 60 minutes, add purification to the crystallizer Water (conductivity is 3.6μs / cm), flow rate is 2.0mL / min, when the mass fraction of water in the solution is 30%, stop feeding purified water. Then add activated carbon for decolorization and filter. Then move the filtrate into the crystallizer and keep it warm at 40°C, introduce ultrasonic wave to induce crystallization, the ultrasonic frequency is 40KHz, cool down to 20°C within 200 minutes and keep it warm for 2 hours to grow crystals; then stop the ultrasonic wave, and cool down to 5°C within 150 minutes , and turn on the ultrasonic wave, the ultrasonic frequency is 33KHz, and grow the crystal for 2h. Then, it was filtered, and the filter cake was washed with water, and dried at 40° C. under normal pressure for 12 hours. The yield of...
Embodiment 2
[0053] Example 2. Add 6g of nifedipine into a crystallizer filled with 100g of methanol and water mixed solvent (mass ratio: 19:1), stir and dissolve at 50°C, and after continuous stirring for 30 minutes, add purification to the crystallizer Water (conductivity is 4.0μs / cm), flow rate is 1.5mL / min, when the mass fraction of water in the solution is 20%, stop feeding purified water. Then add activated carbon for decolorization and filter. Then move the filtrate into the crystallizer and keep it warm at 45°C, introduce ultrasonic wave to induce crystallization, the ultrasonic frequency is 33KHz, cool down to 30°C within 150 minutes and keep it warm for 1 hour to grow the crystal; then stop the ultrasonic wave, and cool down to 10°C within 100 minutes , and turn on the ultrasonic wave, the ultrasonic frequency is 20KHz, and grow the crystal for 4h. Then, it was filtered, and the filter cake was washed with methanol, and dried at 60° C. under normal pressure for 10 hours. The fi...
Embodiment 3
[0054] Example 3. Add 5g of nifedipine into a crystallizer filled with 100g of methanol and water mixed solvent (mass ratio: 9:1), stir and dissolve at 45°C, and after continuous stirring for 40 minutes, add purification to the crystallizer Water (conductivity is 4.5μs / cm), flow rate is 1.0mL / min, when the mass fraction of water in the solution is 25%, stop feeding purified water. Then add activated carbon for decolorization and filter. Then move the filtrate into the crystallizer and keep it warm at 30°C, introduce ultrasonic wave to induce crystallization, the ultrasonic frequency is 20KHz, cool down to 20°C within 60 minutes and keep it warm for 1 hour to grow the crystal; then stop the ultrasonic wave, and cool down to 10°C within 100 minutes , and turn on the ultrasonic wave, the ultrasonic frequency is 25KHz, and grow the crystal for 3h. Then, it was filtered, and the filter cake was washed with ethanol, and dried at 50° C. under normal pressure for 8 hours. The final ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com