Anionic polymerization method and method for producing polymer
An anionic polymerization and compound technology, applied in the field of polymer manufacturing, can solve problems such as increased cooling costs, unfavorable industrial production, and difficulty in exerting high activity, and achieve the effect of inhibiting gel components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131](1) Set a half-moon-type stir bar in a three-mouth flask of content accumulation, replace the atmosphere in the system. Toluene 28.3 g, 1,1,4,7,10,10-hexamethylene ethyl tetraamine 0.35 g and a three-substrate organic aluminum compound prepared in Reference Example 1 in a concentration of 0.46 mmol / L (A) The toluene solution was 6.5 ml, cooled to 20 ° C with a water bath. 1. 12 ml of cyclohexane containing sec-butyllithium 1.30 mmol was added thereto, stirred for 20 minutes. At this time, the concentration of 1,1,4,7,10,10-hexamethylene ethylenediamine and sec-butyl lithium were 34.1 mmol / kg, respectively. The solution was drastically stirred, and the methyl methacrylate was added dropwise to the methylene methacrylate at 20 ° C. The solution was originally colored as yellow, faded after 1 minute after the end of the drop.
[0132](2) A portion of the solution obtained in the above (1) is sampled, and it is injected into a large amount of methanol, and the precipitated white ...
Embodiment 2~4
[0135]"Example 2 to 4, Comparative Examples 1, 2"
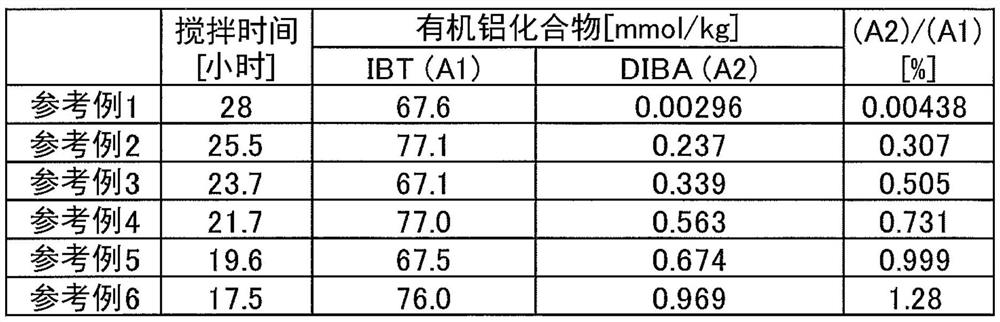
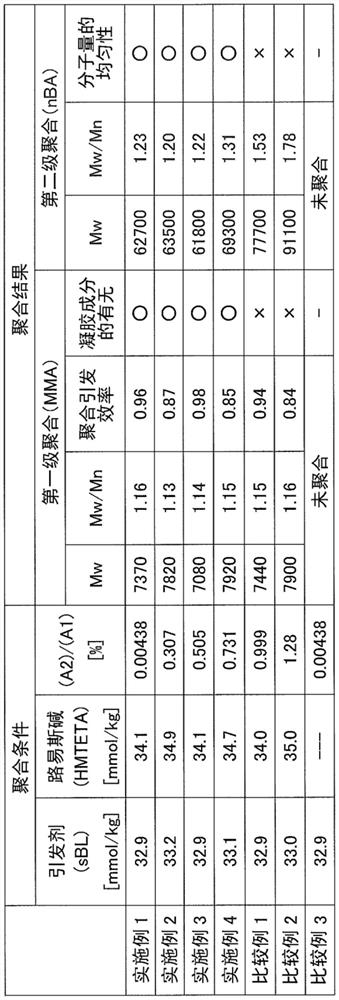
[0136]The three-substituted organoal aluminum compound (a) prepared in Reference Example 1 was changed to the three-substituted organoaluminum compound prepared in Reference Examples 2 to 6, except that the polymerization operation and polymerization stopped in the same manner as in Example 1. operating. The polymerization conditions and polymerization are shown in Table 2 below.
[0137]"Comparative Example 3"
[0138]The use of 1,1,4,7,10,10-hexamethylenedicanethylenedicaine is omitted, and the polymerization operation and aggregation stop operation is performed in the same manner as in Example 1. The polymerization conditions and polymerization are shown in Table 2 below.
[0139][Table 2]
[0140]
[0141]The symbols in Table 2 above have the following meanings.
[0142]SBL: Zhongshiki
[0143]HMTETA: 1,1,4,7,10,10-hexamethylene ethyl tetraamine
[0144]MMA: methyl methacrylate
[0145]NBA: n-butyl acrylate
[0146]From the results shown in Table 2 described a...
Embodiment 5~7
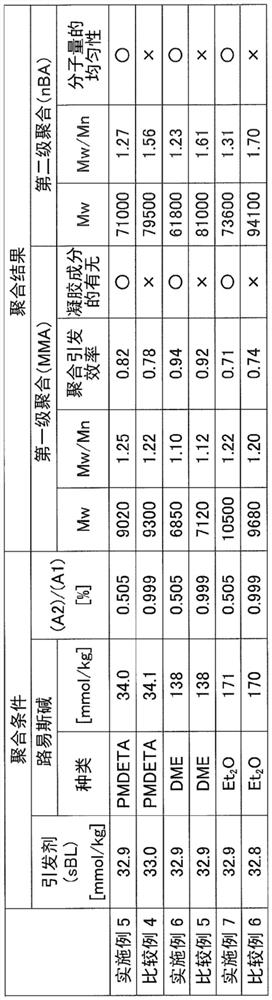
[0149]The use of 1,1,4,7,10,10-hexamethylene tri-methylamine is changed to N, N, N ', N ", N, N", respectively, respectively, respectively, respectively, respectively. 2-dimethoxyethane, or diethyl ether, in the same manner as in Example 3, the polymerization operation and the aggregation stop operation. The polymerization conditions and polymerization are shown in Table 3 below.
[0150]"Comparative Example 4"
[0151]The three-substituted organoal aluminum compound (A) prepared in Reference Example 3 is made to the three-substituted organoal aluminum compound (a) prepared in Reference Example 5, except that the polymerization operation and the aggregation stop operation in the same manner as in Example 5. The polymerization conditions and polymerization are shown in Table 3 below.
[0152]"Comparative Example 5"
[0153]The three-substituted organoal aluminum compound (a) prepared in Reference Example 3 is changed to the three-substituted organoal aluminum compound (a) prepared in Reference E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com