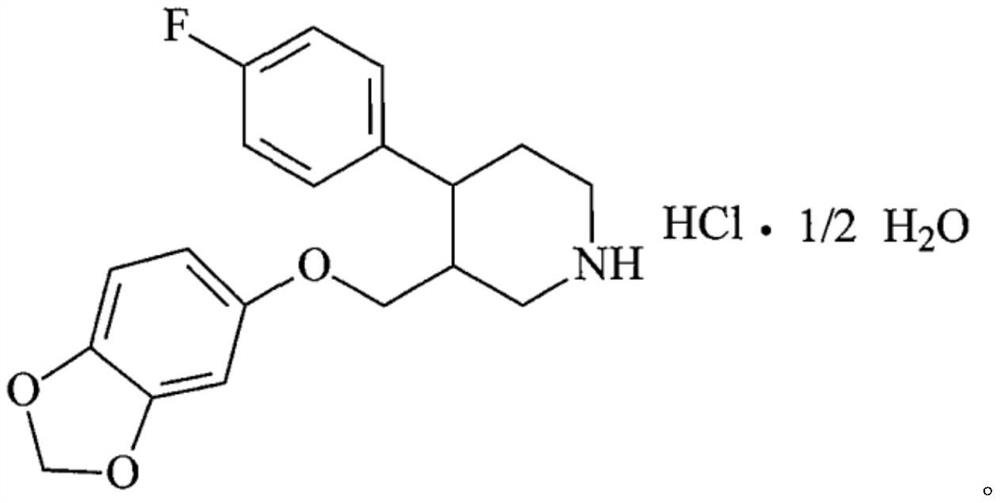
Paroxetine hydrochloride tablet and preparation method thereof
The technology of paroxetine hydrochloride tablets and paroxetine hydrochloride is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., and can solve the problems of being unsuitable for mass production, cumbersome technological process, and high risk of sticking and flushing, Achieve the effect of reducing the risk of uneven content and sticking, simple process flow, and reducing the risk of sticking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
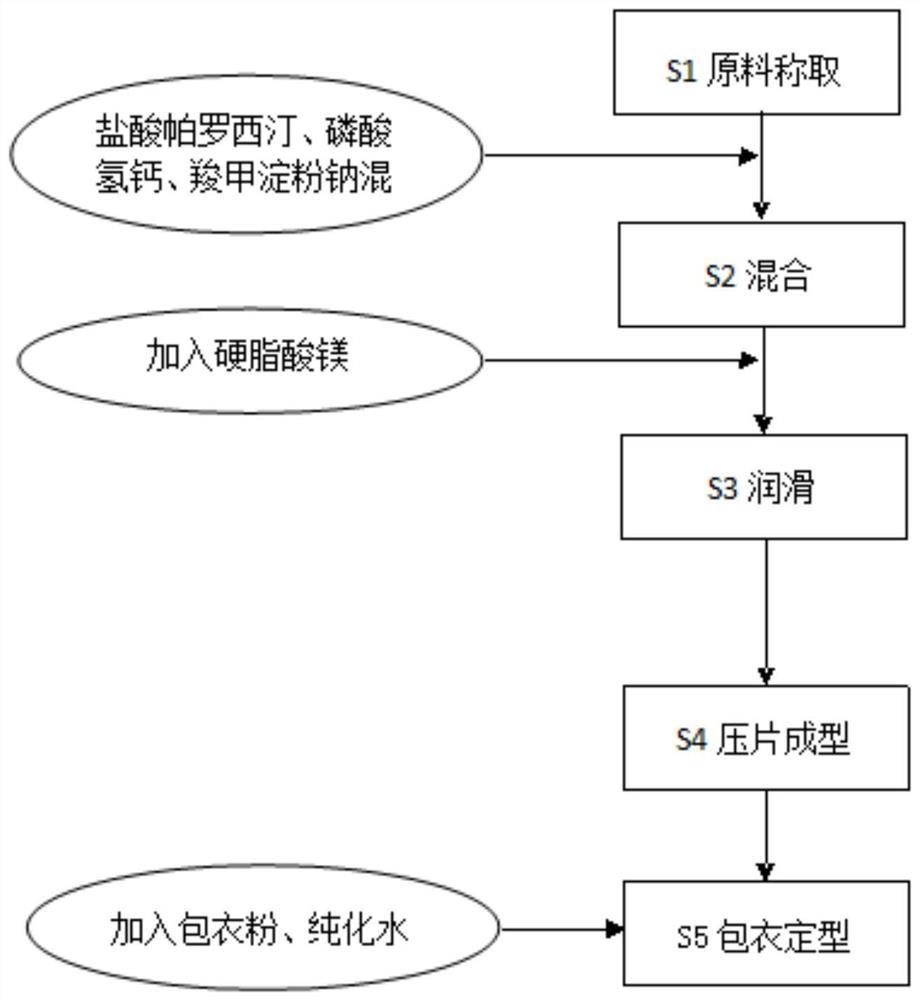
[0037] The present invention also provides a preparation method for preparing the paroxetine hydrochloride tablet, which adopts the preparation method of powder mixing and direct tablet compression, and the preparation method comprises the following steps:
[0038] Weighing of S1 raw materials, successively weighing paroxetine hydrochloride, filler, disintegrant, lubricant and coating powder for later use;
[0039] S2 mixing, put paroxetine hydrochloride, filler, and disintegrating agent into the mixing granulator in sequence and mix evenly. The mixing speed is 1000-2000 rpm, the stirring speed is 50-200 rpm, and the mixing time is 5-15 minutes;
[0040] S3 lubrication, add lubricant, continue to mix evenly, the stirring speed of mixing is 5-20rpm, and the mixing time is 2-15min;
[0041] S4 tablet molding, the uniformly mixed powder is directly compressed into a tablet to obtain a tablet core;
[0042] S5 Coating and shaping, the tablet core is coated with coating powder and...
Embodiment 1
[0045] Prepare paroxetine hydrochloride tablet, every 1000 comprises the component (unit is g) of following weight:
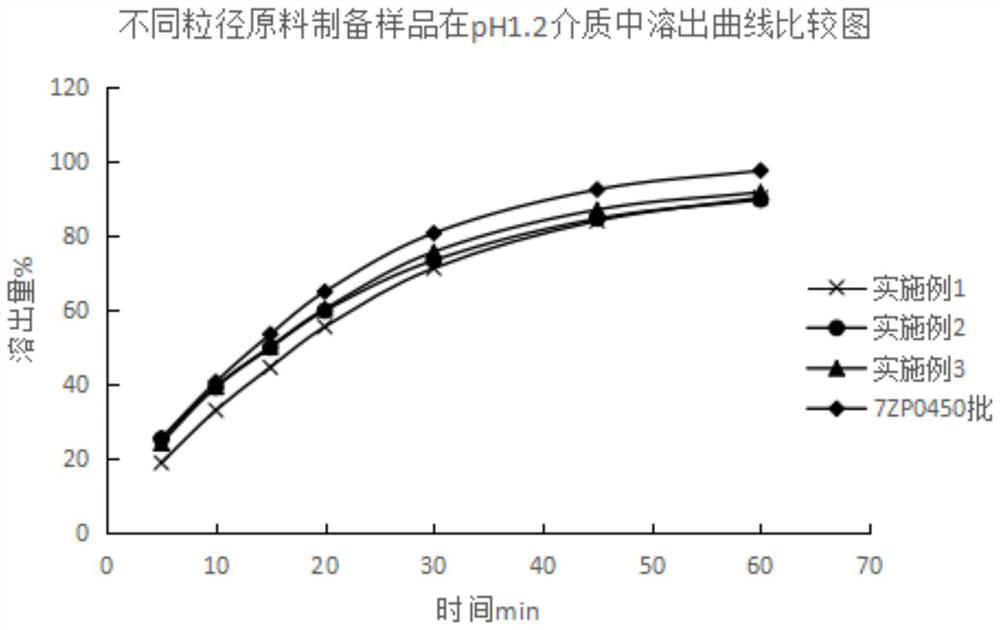
[0046] 22.8g of paroxetine hydrochloride with a D90 particle size of 9μm≤D90≤15μm, 318.0g of calcium hydrogen phosphate (D90≤200μm), 6.0g of sodium carboxymethyl starch, 3.2g of magnesium stearate, and 7g of coating powder;
[0047] The paroxetine hydrochloride tablet adopts the powder mixing direct compression preparation method, and the preparation method comprises the following steps:
[0048] Weigh the raw materials of S1, and successively weigh paroxetine hydrochloride, calcium hydrogen phosphate, sodium carboxymethyl starch, magnesium stearate and coating powder for later use;
[0049] S2 Mixing, put paroxetine hydrochloride, calcium hydrogen phosphate, sodium carboxymethyl starch into the mixing granulator in turn and mix evenly, the mixing speed of chopping is 1000rpm, the stirring speed is 50rpm, and the mixing time is 15min;
[0050] S3 lubrication, ad...
Embodiment 2
[0054] Prepare paroxetine hydrochloride tablet, every 1000 comprises the component (unit is g) of following weight:
[0055] 22.8g of paroxetine hydrochloride with a D90 particle size of 33μm≤D90≤40μm, 318.0g of calcium hydrogen phosphate (D90≤200μm), 6.0g of sodium carboxymethyl starch, 3.2g of magnesium stearate, and 7g of coating powder;
[0056] The paroxetine hydrochloride tablet adopts the powder mixing direct compression preparation method, and the preparation method comprises the following steps:
[0057] The chopping speed of S2 mixing is 1500rpm, the stirring speed is 100rpm, and the mixing time is 8min;
[0058] The stirring speed of S3 mixing is 10rpm, and the mixing time is 8min;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com