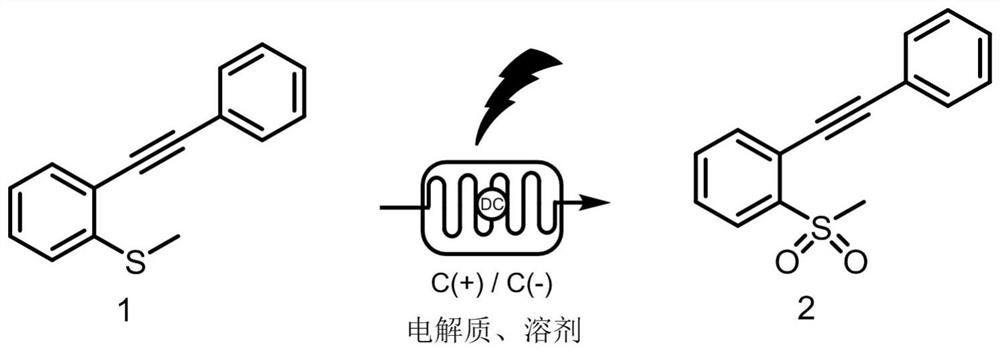
Method for continuously preparing 1-(methylsulfonyl)-2-(phenyl ethynyl) benzene by adopting electrochemical micro-channel
A technology of methylsulfonyl and phenylacetylene, applied in the direction of electrolytic components, electrolytic process, electrolytic organic production, etc., can solve the problems of using strong oxidant and long reaction process cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthesis of 1-(methylsulfonyl)-2-(phenylethynyl)benzene:
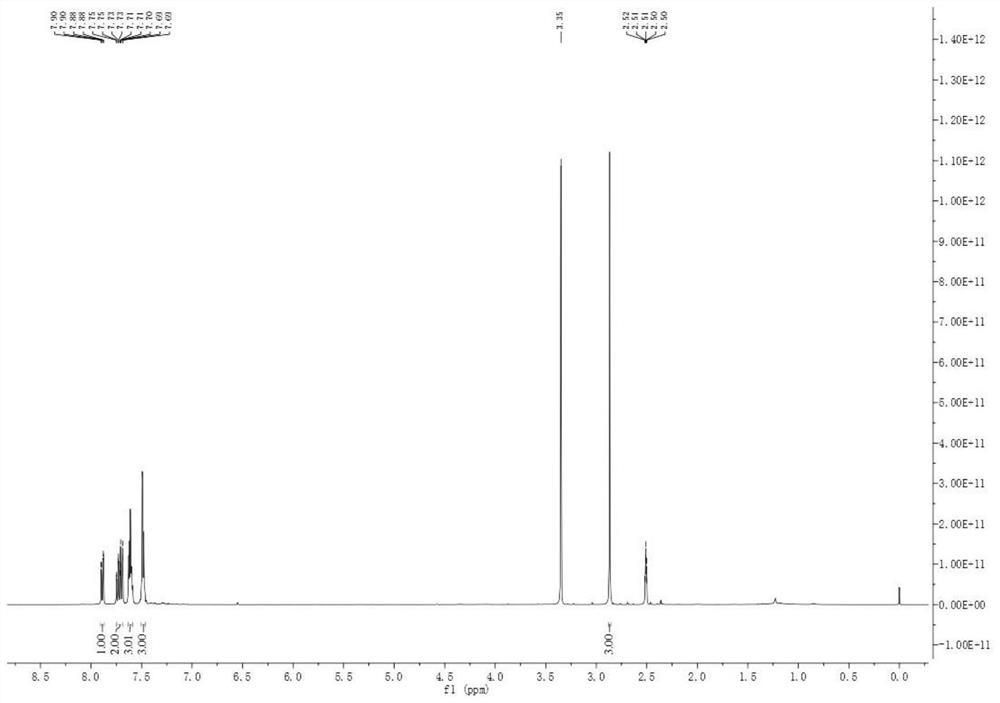
[0040] Dissolve 0.25 mmol (0.056 g) of compound 1 and 0.5 mmol of sodium acetate (0.041 g) in a mixed solution of 10 mL of ethanol and water (ethanol:water=4:1, that is, 8 mL of ethanol and 2 mL of water), The homogeneous solution A was obtained and added to the syringe pump a; the injection flow rate of the syringe pump a was 225 mL / min; the current was set to 10 mA, and the temperature was room temperature; the reaction volume of the microchannel reactor was V = 225 mL, and the reaction time was 1 min After a period of reaction in the microchannel reactor, the reaction liquid is collected, and the product yield calculated by HPLC is 90%, and the reaction liquid, after washing, drying and filtering, obtains the product 1-(formazan) after column chromatography separation sulfonyl)-2-(phenylethynyl)benzene. Such as image 3 with Figure 4 as shown, 1H NMR (400 MHz, DMSO- d 6 ) δ7.89 (dd, J = 7....
Embodiment 2
[0041] Example 2 Synthesis of 1-(methylsulfonyl)-2-(phenylethynyl)benzene:
[0042] Dissolve 0.25 mmol (0.056 g) of compound 1 and 0.5 mmol sodium acetate (0.041 g) in a mixed solution of 10 mL of acetonitrile and water (acetonitrile: water = 4:1, that is, 8 mL of acetonitrile and 2 mL of water), The homogeneous solution A was obtained and added to the syringe pump a; the injection flow rate of the syringe pump a was 225 mL / min; the current was set to 10 mA, and the temperature was room temperature; the reaction volume of the microchannel reactor was V = 225 mL, and the reaction time was 1 min After a period of reaction in the microchannel reactor, the reaction liquid was collected, and the product yield was calculated as 50% by the method of HPLC, and the reaction liquid was washed with water, dried, filtered, and obtained product 1-(formazol) after column chromatography separation sulfonyl)-2-(phenylethynyl)benzene.
Embodiment 3
[0043] Example 3 Synthesis of 1-(methylsulfonyl)-2-(phenylethynyl)benzene:
[0044] Dissolve 0.25 mmol (0.056 g) of compound 1 and 0.5 mmol sodium acetate (0.041 g) in a mixed solution of 10 mL of trifluoroethanol and water (trifluoroethanol:water=4:1, that is, 8 mL of trifluoroethanol and 2 mL of water) to obtain a homogeneous solution A, which was added to syringe pump a; the injection flow rate of syringe pump a was 225 mL / min; the current was set to 10 mA, and the temperature was room temperature; the microchannel reactor reaction volume V = 225 mL, reaction time 1 min; after a period of reaction in the microchannel reactor, the reaction liquid was collected, and the product yield was calculated by HPLC as 68%. The reaction liquid was washed with water, dried, filtered, and separated by column chromatography The product 1-(methylsulfonyl)-2-(phenylethynyl)benzene was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com