Application of Lactobacillusparacasei L.pR3-10 in preparation of drug for relieving anxiety and improving sleep
A technology to improve sleep and Lactobacillus, applied in the field of microorganisms, can solve the problems of lack of scientific research evidence on the function of probiotics, not necessarily suitable, and few probiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1L
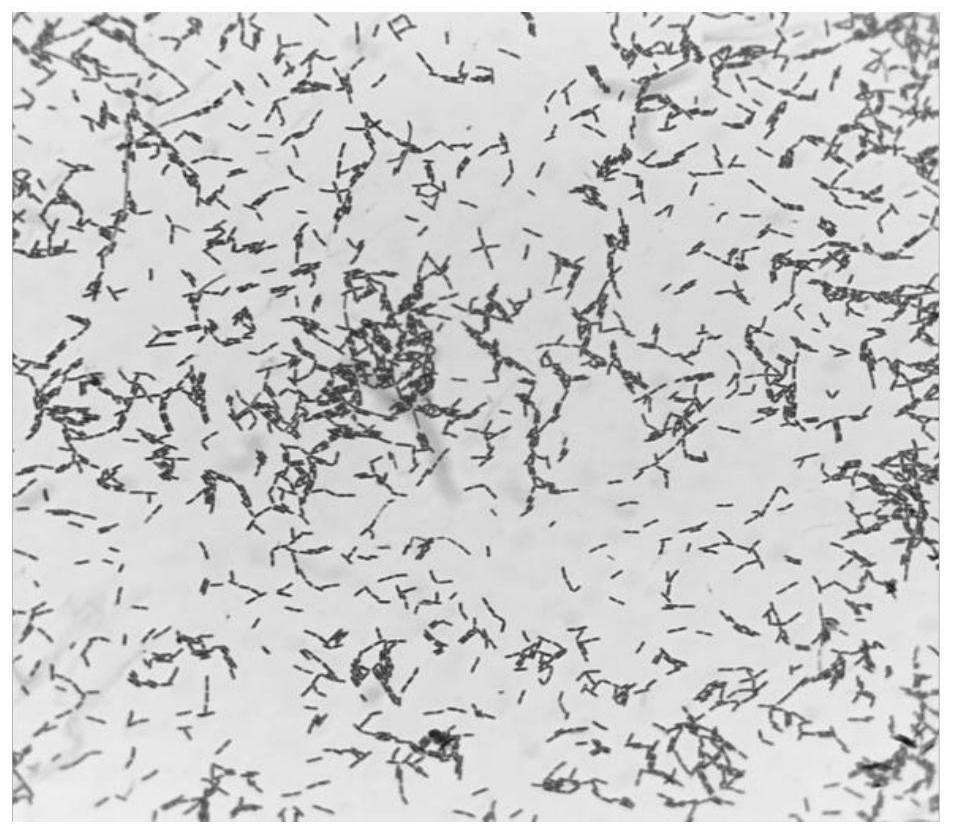
[0024] The isolation and identification of the precursor strain L.p R3 (Lactobacillus paracasei) of embodiment 1L.p R3-10
[0025] (1) Sample source
[0026] Healthy babies aged 0-6 months were selected from the families of school staff as volunteers. Two weeks before sampling, a normal diet is required and there is no recent history of intestinal infection or antibiotic use. Take the morning stool on the day of sampling, and quickly use the intelligent microbial separation system of Nanjing Famet Company to separate the fecal bacteria after collection. After the separation was completed, the crude fecal bacteria liquid was collected quickly, added with cryopreservation protection solution, and then stored in a -80°C ultra-low temperature refrigerator for future use.
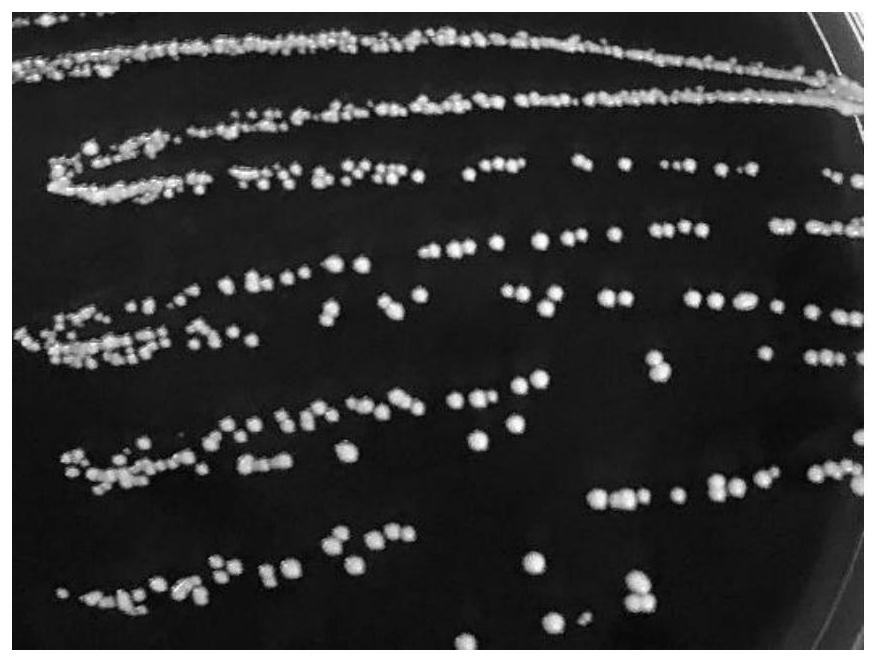
[0027] (2) Isolation, culture and identification of L.p R3
[0028] Take 1mL of the crude fecal extract liquid and add it to 9mL of normal saline, mix well and perform gradient dilution. Draw the dilution con...
Embodiment 2L
[0031] Induction and identification of embodiment 2L.p R3-10
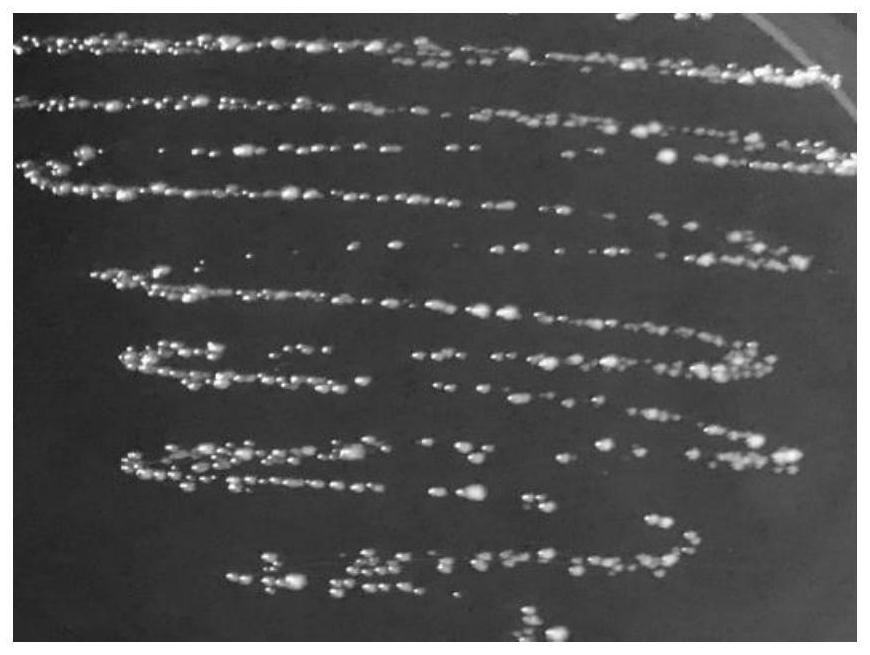
[0032] (1) Low nutrient gradient tolerance method induces L.p R3 to become L.p R3-10
[0033]Take out the frozen L.p R3 from the -80°C refrigerator and put it in a warm water bath at 37°C to melt it quickly. The thawed bacterial solution was poured into an anaerobic blood agar plate, and placed in an anaerobic condition at 37°C for static culture for 48 hours. Observe the growth of the colony on the plate and whether there is a hemolytic ring, observe the shape of the strain under a Gram staining microscope, and confirm that there is no pollution, transfer to the MRS agar plate, culture it under anaerobic conditions at 37°C for 24 hours, and pick a single cell on the plate. The colonies were inoculated in 6mL MRS liquid medium, and cultured and activated under anaerobic conditions at 37°C for 16-18h. The activated bacterial solution was inoculated into 100 mL of MRS broth with an inoculum amount of 3% (v / v), cult...
Embodiment 3
[0043] Preparation of embodiment 3 Lactobacillus paracasei L.p R3-10 fermentation supernatant (extracellular secretion), bacterium suspension (thalline)
[0044] Lactobacillus paracasei L.p R3-10 was activated and cultured and inoculated in MRS liquid medium. After culturing at 37°C for 15 hours, the concentration of fermentation bacteria was adjusted to 1×10 7 CFU / mL, centrifuged at 4°C, 6000r / min for 10min to obtain the culture supernatant and cell pellet, the supernatant was filtered through a 0.22μm filter membrane to obtain the fermentation supernatant (extracellular secretion); the cell pellet was filtered through PBS twice After the first wash, the cells were resuspended with PBS, and the cell concentration was adjusted to 1×10 7 CFU / mL to obtain bacterial suspension (bacteria). Fermentation supernatant (extracellular secretions) and bacterial suspension (bacteria) were heated at 121°C for 20 minutes to prepare heat-inactivated fermentation supernatant (extracellular s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com