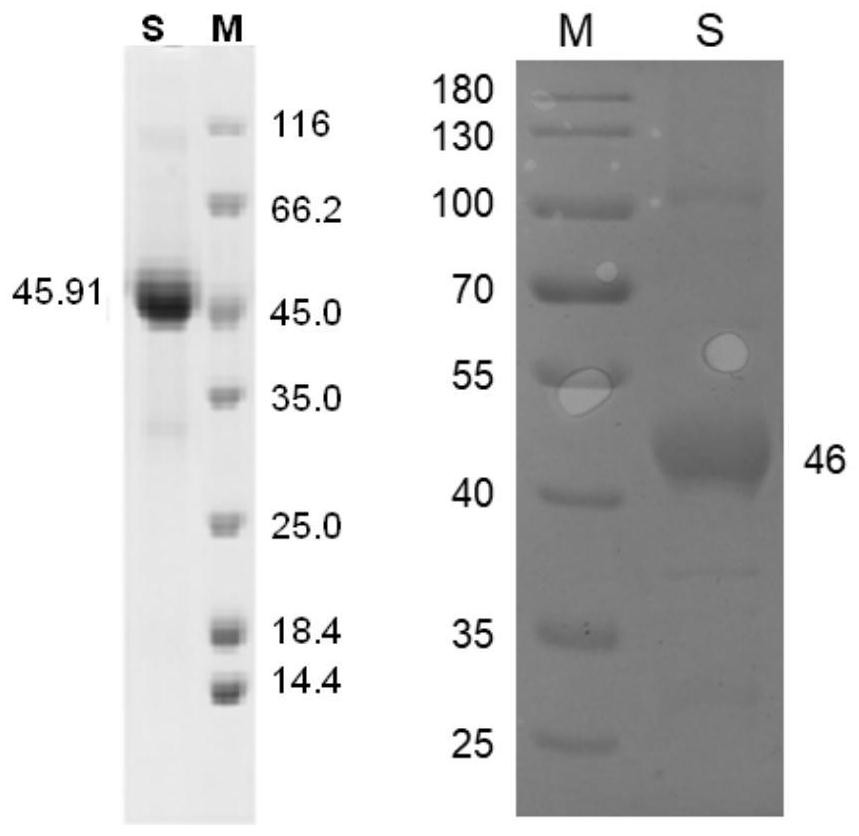
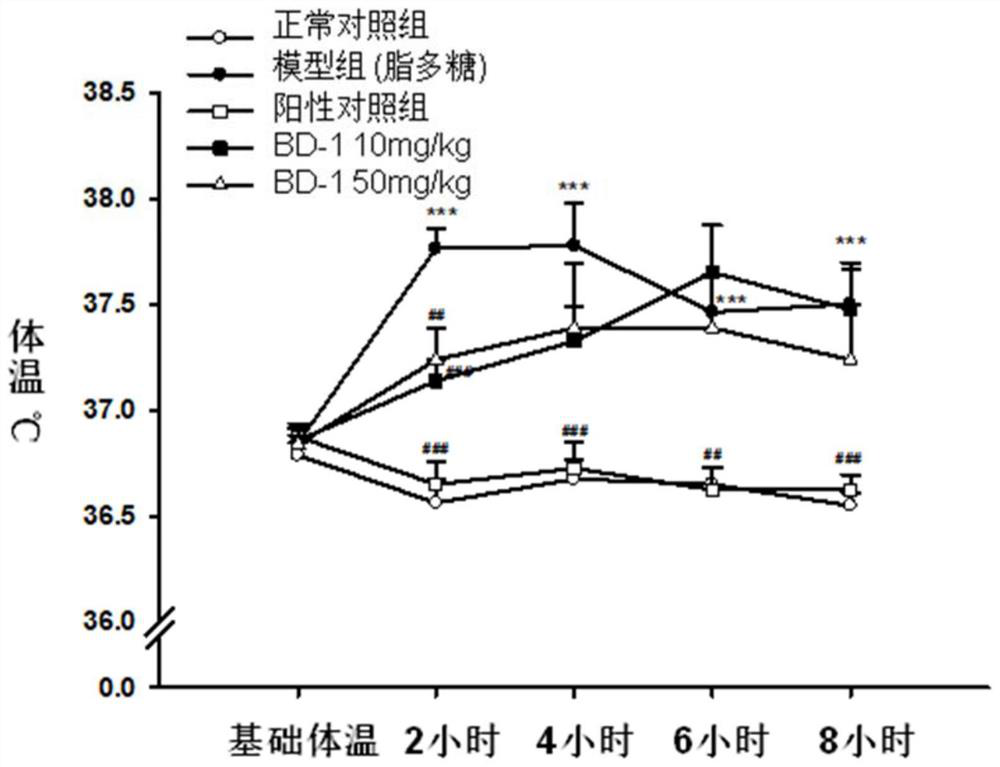
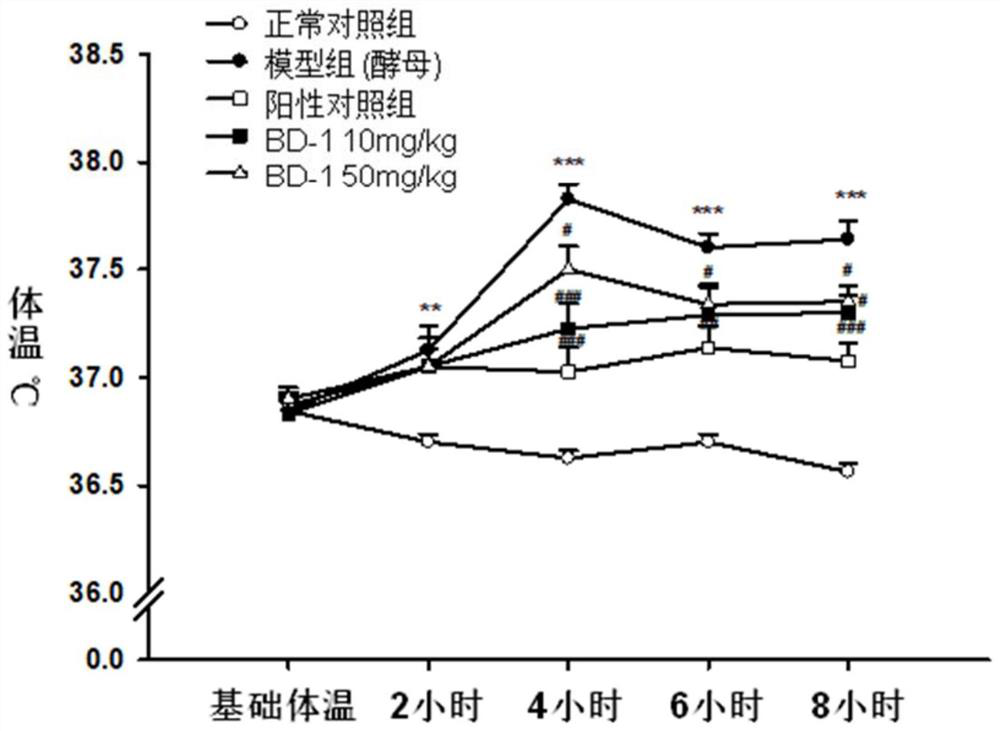
Keratin BD-1, preparation method, pharmaceutical composition and application thereof
A technology of BD-1 and keratin, which is applied in the field of keratin BD-1, its preparation method and its pharmaceutical composition and application, and achieves the effects of high yield, strong effect and inhibition of body temperature rise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115]Example 1 Shake flask fermentation Preparation Protein BD-1 Crude Solution A (TB medium)
[0116]The nucleotide sequence shown in SEQ ID No. 2 is synthesized into a PET-28A (+) vector; sequencing determines the expression vector containing the correct sequence; transfection of the expression vector into BL21 (DE3) cells, The expression sensation-sensitive host cell containing the target nucleotide sequence was obtained. In the lB medium, in the shaker, it was cultured at 37 ° C, 220 rpm, and the recombinant strain was obtained.
[0117]The recombinant strain is pulled out in LBA flat line containing Kanamamycin, and the plate is inverted at a constant temperature incubator overnight for 16 hours overnight.
[0118]Configure 400 ml TB medium, 2 bottles, 200ml per bottle. Kanamamin (final concentration 50 μg / ml) was added to each bottle (200 mL), and a single column on the plate was added to Tb medium, in the shaker, overnight amplification of 37 ° C, 220 rpm. Got seed fluid.
[0119]The ...
Embodiment 2
[0124]Example 2 Shake flask fermentation Preparation Protein BD-1 Crude Solution B (Other Media)
[0125]The synthesis and sequencing determination of the expression vector containing the sequence shown in SEQ ID No. 2 is determined; the expression vector is transfected into Transsetta (DE3) cells to obtain an expression sensitive host cell containing the target nucleotide sequence.
[0126]20 ml of LB medium was prepared, 800 μL was added to 50 μl of host cells containing the target coding sequence, in the shaker, under 37 ° C, 220 rpm, and cultured for 1 hour.
[0127]The above bacteria was taken in LBA plate contained in Kanamamycin, and the plate was inverted at a constant temperature incubator overnight for 16 hours in the 37 ° C.
[0128]10 ml of Lb medium was taken, and Kanamamicin (final concentration 50 μg / ml) was added, and a single bacterium on the plate was added to the LB medium, in the shaker, overnight amplification under 37 ° C, 220 rpm, and cultured for 15 hours, resulting in...
Embodiment 3
[0134]Example 3 Fermentation tank Preparation Protein BD-1 Crude Solution C
[0135]The synthesis and sequencing determination of the expression vector containing the sequence shown in SEQ ID No. 2 is determined; the expression vector is transfected into BL21 (DE3) cells to obtain an expression sensation-sensitive host cell containing the target nucleotide sequence. In the lB medium, in the shaker, it was cultured at 37 ° C, 220 rpm, and the recombinant strain was obtained.
[0136]In LBA plates containing Kanamamicin, 100 μl of recombinant strains was added, and the coater was applied to uniform, and the plate was inverted at a constant temperature incubator overnight culture in a 37 ° C. Take three single collapses in a flat-panel in Kanamamycin, the plate overnight culture, and after three batches of shake flask fermentation expression verify that the strain was stored in 15% glycerol, it was separated into each 0.8ml, which was obtained. Working cell library, freezed to -80 ° C refrig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com